Feasibility of Microbubble-Accelerated Low-Dose Thrombolysis of Peripheral Arterial Occlusions Using an Ultrasound Catheter
- PMID: 36172738
- PMCID: PMC11110464
- DOI: 10.1177/15266028221126938
Feasibility of Microbubble-Accelerated Low-Dose Thrombolysis of Peripheral Arterial Occlusions Using an Ultrasound Catheter
Abstract
Purpose: Intra-arterial administration of microbubbles (MBs) through an ultrasound (US) catheter increases the local concentration of MBs into the thrombus and may further enhance outcomes of contrast-enhanced sonothrombolysis (CEST). The objective of this study was to evaluate the feasibility and lytic efficacy of intra-arterial infusion of MBs during US-enhanced thrombolysis in both in vitro and in vivo peripheral arterial occluded models.
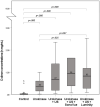
Materials and methods: SonoVue and Luminity MBs were infused at a flow rate of 20 mL/h through either the drug delivery lumen of the US catheter (DDC, n=20) or through the tube lumen of the vascular phantom (systematic infusion, n=20) during thrombolysis with a low-dose urokinase (UK) protocol (50 000 IU/h) with(out) US application to assess MB survivability and size by pre-treatment and post-treatment measurements. A human thrombus was placed into a vascular phantom of the flow system to examine the lytic effects of CEST by post-treatment D-dimer concentrations measurements of 5 treatment conditions (saline, UK, UK+US, UK+US+SonoVue, and UK+US+Luminity). Thrombolytic efficacy of localized MBs and US delivery was then investigated in vivo in 5 porcine models by arterial blood flow, microcirculation, and postmortem determined thrombus weight and remaining length.
Results: US exposure significantly decreased SonoVue (p=0.000) and Luminity (p=0.000) survivability by 37% and 62%, respectively. In vitro CEST treatment resulted in higher median D-dimer concentrations for the SonoVue (0.94 [0.07-7.59] mg/mL, p=0.025) and Luminity (0.83 [0.09-2.53] mg/mL, p=0.048) subgroups when compared with thrombolysis alone (0.36 [0.02-1.00] mg/mL). The lytic efficacy of CEST examined in the porcine model showed an improved median arterial blood flow of 21% (7%-79%), and a median thrombus weight and length of 1.02 (0.96-1.43) g and 2.25 (1.5-4.0) cm, respectively. One allergic reaction and 2 arrhythmias were observed due to the known allergic reaction on lipids in the porcine model.
Conclusion: SonoVue and Luminity can be combined with an US catheter and could potentially accelerate thrombolytic treatment of peripheral arterial occlusions.
Clinical impact: Catheter-directed thrombolysis showed to be an effective alternative to surgery for acute peripheral arterial occlusions, but this technique is still associated with several limb and life-threatening complications. The effects of thrombolysis on clot dissolution may be further enhanced by intra-arterial administration of microbubbles through an ultrasound catheter. This study demonstrates the feasibility and lytic efficacy of intra-arterial infusion of microbubbles during US-enhanced thrombolysis in both in vitro and in vivo peripheral arterial occluded models.
Keywords: contrast-enhanced sonothrombolysis; drug delivery; endovascular treatment/therapy; in vitro; in vivo; microbubbles; peripheral artery disease; thrombolysis; ultrasound.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The in vitro study was supported by an unrestricted research grant from LamePro B.V. and Bracco International B.V. Both corporations had no role in the study design, collection, analysis, and interpretation of data, nor in writing of the report, submitting the report for publication, or ultimate authority on any of the previous mentioned.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

