Hospital Variation of Spironolactone Use in Patients Hospitalized for Heart Failure in China-The China PEACE Retrospective Heart Failure Study
- PMID: 36172964
- PMCID: PMC9673705
- DOI: 10.1161/JAHA.122.026300
Hospital Variation of Spironolactone Use in Patients Hospitalized for Heart Failure in China-The China PEACE Retrospective Heart Failure Study
Abstract
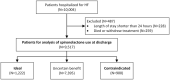
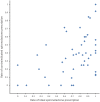
Background Although aldosterone antagonists improve outcomes in select individuals with heart failure and reduced ejection fraction, studies in the United States have raised concerns about underuse and overuse. Variations in the prescription of aldosterone antagonist in China are unknown. Methods and Results In the multicenter, hospital-based, retrospective China PEACE (China Patient-Centered Evaluative Assessment of Cardiac Events) study, we identified a nationally representative cohort of admissions for heart failure in a nationally representative sample of Chinese hospitals in 2015. Patients were classified into 1 of 3 groups according to their eligibility for spironolactone-"ideal" (left ventricular ejection fraction <40% and without contraindications), "contraindicated" (a documented contraindication, irrespective of left ventricular ejection fraction), and "uncertain-benefit" (all others). We measured hospital variation of spironolactone prescriptions at discharge in the "ideal" and "contraindicated" group and calculated the median odds ratio (MOR), a measure of institution-level variation for 2 individuals with similar characteristics discharged at 2 randomly selected hospitals. Hospital characteristics associated with spironolactone use were identified using multivariable linear regression model. Among 1222 ideal patients from 97 hospitals, the median rate of spironolactone prescription was 78.6% (interquartile range [IQR], 42.8%-89.6% [range, 0%-100%], MOR, 3.4 [95% CI, 2.7-4.0]) at discharge. Among 900 contraindicated patients from 83 hospitals, the median rate of spironolactone prescription was 30.0% (IQR, 9.1%-50.0% [range, 0%-100%], MOR, 3.1 [95% CI, 2.4-3.9]) at discharge. Hospitals with independent departments of cardiology and located in Eastern China were associated with a 38.0% (95% CI, 18.7-57.3; P<0.001) and a 14.6% (95% CI, 2.3%-26.9%; P=0.020) higher rate of spironolactone use for ideal patients. Conclusions In this national study of hospitals in China, the use of spironolactone among ideal patients and the inappropriate use of spironolactone among patients with contraindications was substantial, with rates that varied markedly by institution. Registration URL: https://www.clinicaltrials.gov. Unique identifier: NCT02877914.
Keywords: China; heart failure; quality of care; spironolactone.
Figures

Similar articles
-
National quality assessment evaluating spironolactone use during hospitalization for acute myocardial infarction (AMI) in China: China Patient-centered Evaluation Assessment of Cardiac Events (PEACE)-Retrospective AMI Study, 2001, 2006, and 2011.J Am Heart Assoc. 2015 Jun 12;4(6):e001718. doi: 10.1161/JAHA.114.001718. J Am Heart Assoc. 2015. PMID: 26071031 Free PMC article.
-
Temporal Trends and Hospital Variation in Mineralocorticoid Receptor Antagonist Use in Veterans Discharged With Heart Failure.J Am Heart Assoc. 2015 Dec 23;4(12):e002268. doi: 10.1161/JAHA.115.002268. J Am Heart Assoc. 2015. PMID: 26702082 Free PMC article.
-
Characteristics, Management, and Outcomes of Patients Hospitalized for Heart Failure in China: The China PEACE Retrospective Heart Failure Study.J Am Heart Assoc. 2019 Sep 3;8(17):e012884. doi: 10.1161/JAHA.119.012884. Epub 2019 Aug 21. J Am Heart Assoc. 2019. PMID: 31431117 Free PMC article.
-
[Effect of spironolactone in patients with heart failure and preserved left ventricular function - TOPCAT study].Vnitr Lek. 2015 May;61(5):376-80. Vnitr Lek. 2015. PMID: 26075843 Review. Czech.
-
Spironolactone for Management of Heart Failure with Preserved Ejection Fraction: Whither to After TOPCAT?Curr Atheroscler Rep. 2015 Nov;17(11):64. doi: 10.1007/s11883-015-0541-6. Curr Atheroscler Rep. 2015. PMID: 26408016 Review.
Cited by
-
Impact of Standardized Heart Failure Management Center Construction on the Management of Patients With Chronic Heart Failure.Clin Cardiol. 2025 Jan;48(1):e70076. doi: 10.1002/clc.70076. Clin Cardiol. 2025. PMID: 39780449 Free PMC article.
References
-
- Heart Failure Group of Chinese Society of Cardiology of Chinese Medical Association; Chinese Heart Failure Association of Chinese Medical Doctor Association; Editorial Board of Chinese Journal of Cardiology . [Chinese guidelines for the diagnosis and treatment of heart failure 2018]. Zhonghua Xin Xue Guan Bing Za Zhi. 2018 Oct 24;46(10):760–789. Chinese. doi: 10.3760/cma.j.issn.0253-3758.2018.10.004 - DOI - PubMed
-
- Writing Committee Members , Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines , et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776 - DOI - PubMed
-
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone evaluation study investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

