Potential Therapeutic Effects of Short-Chain Fatty Acids on Chronic Pain
- PMID: 36173071
- PMCID: PMC10788890
- DOI: 10.2174/1570159X20666220927092016
Potential Therapeutic Effects of Short-Chain Fatty Acids on Chronic Pain
Abstract
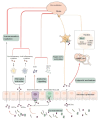
The intestinal homeostasis maintained by the gut microbiome and relevant metabolites is essential for health, and its disturbance leads to various intestinal or extraintestinal diseases. Recent studies suggest that gut microbiome-derived metabolites short-chain fatty acids (SCFAs) are involved in different neurological disorders (such as chronic pain). SCFAs are produced by bacterial fermentation of dietary fibers in the gut and contribute to multiple host processes, including gastrointestinal regulation, cardiovascular modulation, and neuroendocrine-immune homeostasis. Although SCFAs have been implicated in the modulation of chronic pain, the detailed mechanisms that underlie such roles of SCFAs remain to be further investigated. In this review, we summarize currently available research data regarding SCFAs as a potential therapeutic target for chronic pain treatment and discuss several possible mechanisms by which SCFAs modulate chronic pain.
Keywords: Short-chain fatty acids; chronic pain; gut microbiome; gut-brain communication; intestinal diseases.; metabolites.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Conflict of interest statement
The authors declare no conflict of interest, financial or otherwise.
Figures
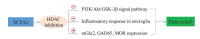

Similar articles
-
Short Chain Fatty Acids: Fundamental mediators of the gut-lung axis and their involvement in pulmonary diseases.Chem Biol Interact. 2022 Dec 1;368:110231. doi: 10.1016/j.cbi.2022.110231. Epub 2022 Oct 23. Chem Biol Interact. 2022. PMID: 36288778 Review.
-
Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations.J Physiol. 2018 Oct;596(20):4923-4944. doi: 10.1113/JP276431. Epub 2018 Aug 28. J Physiol. 2018. PMID: 30066368 Free PMC article.
-
Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs).Acta Biochim Pol. 2019 Mar 4;66(1):1-12. doi: 10.18388/abp.2018_2648. Acta Biochim Pol. 2019. PMID: 30831575 Review.
-
Recent advances in the therapeutic application of short-chain fatty acids (SCFAs): An updated review.Crit Rev Food Sci Nutr. 2022;62(22):6034-6054. doi: 10.1080/10408398.2021.1895064. Epub 2021 Mar 11. Crit Rev Food Sci Nutr. 2022. PMID: 33703960 Review.
-
Intestinal Microbiota-Derived Short Chain Fatty Acids in Host Health and Disease.Nutrients. 2022 May 9;14(9):1977. doi: 10.3390/nu14091977. Nutrients. 2022. PMID: 35565943 Free PMC article. Review.
Cited by
-
Unlocking Cardioprotective Potential of Gut Microbiome: Exploring Therapeutic Strategies.J Microbiol Biotechnol. 2024 Dec 28;34(12):2413-2424. doi: 10.4014/jmb.2405.05019. Epub 2024 Jun 25. J Microbiol Biotechnol. 2024. PMID: 39467697 Free PMC article. Review.
-
Gut microbiota-mediated pain sensitization: mechanisms and therapeutic implications.Front Pain Res (Lausanne). 2025 Jul 3;6:1626515. doi: 10.3389/fpain.2025.1626515. eCollection 2025. Front Pain Res (Lausanne). 2025. PMID: 40678181 Free PMC article. Review.
-
Genetically predicted causal effects of gut microbiota on spinal pain: a two-sample Mendelian randomization analysis.Front Microbiol. 2024 Mar 25;15:1357303. doi: 10.3389/fmicb.2024.1357303. eCollection 2024. Front Microbiol. 2024. PMID: 38591041 Free PMC article.
-
Global Trends in Research of Pain-Gut-Microbiota Relationship and How Nutrition Can Modulate This Link.Nutrients. 2023 Aug 24;15(17):3704. doi: 10.3390/nu15173704. Nutrients. 2023. PMID: 37686738 Free PMC article.
-
Role of Vitamin D Status and Alterations in Gut Microbiota Metabolism in Fibromyalgia-Associated Chronic Inflammatory Pain.Biomedicines. 2025 Jan 9;13(1):139. doi: 10.3390/biomedicines13010139. Biomedicines. 2025. PMID: 39857723 Free PMC article.
References
-
- Bonomo R.R., Cook T.M., Gavini C.K., White C.R., Jones J.R., Bovo E., Zima A.V., Brown I.A., Dugas L.R., Zakharian E., Aubert G., Alonzo F., III, Calcutt N.A., Mansuy-Aubert V. Fecal transplantation and butyrate improve neuropathic pain, modify immune cell profile, and gene expression in the PNS of obese mice. Proc. Natl. Acad. Sci. USA. 2020;117(42):26482–26493. doi: 10.1073/pnas.2006065117. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

