Concordance of SARS-CoV-2 Antibody Results during a Period of Low Prevalence
- PMID: 36173112
- PMCID: PMC9599436
- DOI: 10.1128/msphere.00257-22
Concordance of SARS-CoV-2 Antibody Results during a Period of Low Prevalence
Abstract
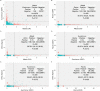
Accurate, highly specific immunoassays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are needed to evaluate seroprevalence. This study investigated the concordance of results across four immunoassays targeting different antigens for sera collected at the beginning of the SARS-CoV-2 pandemic in the United States. Specimens from All of Us participants contributed between January and March 2020 were tested using the Abbott Architect SARS-CoV-2 IgG (immunoglobulin G) assay (Abbott) and the EuroImmun SARS-CoV-2 enzyme-linked immunosorbent assay (ELISA) (EI). Participants with discordant results, participants with concordant positive results, and a subset of concordant negative results by Abbott and EI were also tested using the Roche Elecsys anti-SARS-CoV-2 (IgG) test (Roche) and the Ortho-Clinical Diagnostics Vitros anti-SARS-CoV-2 IgG test (Ortho). The agreement and 95% confidence intervals were estimated for paired assay combinations. SARS-CoV-2 antibody concentrations were quantified for specimens with at least two positive results across four immunoassays. Among the 24,079 participants, the percent agreement for the Abbott and EI assays was 98.8% (95% confidence interval, 98.7%, 99%). Of the 490 participants who were also tested by Ortho and Roche, the probability-weighted percentage of agreement (95% confidence interval) between Ortho and Roche was 98.4% (97.9%, 98.9%), that between EI and Ortho was 98.5% (92.9%, 99.9%), that between Abbott and Roche was 98.9% (90.3%, 100.0%), that between EI and Roche was 98.9% (98.6%, 100.0%), and that between Abbott and Ortho was 98.4% (91.2%, 100.0%). Among the 32 participants who were positive by at least 2 immunoassays, 21 had quantifiable anti-SARS-CoV-2 antibody concentrations by research assays. The results across immunoassays revealed concordance during a period of low prevalence. However, the frequency of false positivity during a period of low prevalence supports the use of two sequentially performed tests for unvaccinated individuals who are seropositive by the first test. IMPORTANCE What is the agreement of commercial SARS-CoV-2 immunoglobulin G (IgG) assays during a time of low coronavirus disease 2019 (COVID-19) prevalence and no vaccine availability? Serological tests produced concordant results in a time of low SARS-CoV-2 prevalence and no vaccine availability, driven largely by the proportion of samples that were negative by two immunoassays. The CDC recommends two sequential tests for positivity for future pandemic preparedness. In a subset analysis, quantified antinucleocapsid and antispike SARS-CoV-2 IgG antibodies do not suggest the need to specify the antigen targets of the sequential assays in the CDC's recommendation because false positivity varied as much between assays targeting the same antigen as it did between assays targeting different antigens.
Keywords: IgG antibodies; SARS-CoV-2; low prevalence; nucleocapsid protein; spike protein.
Conflict of interest statement
The authors declare a conflict of interest. This project has been funded in whole or in part with Federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261201500003I and 75N91019D00024. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. K.A.G. performed this work while serving as the Chief Medical and Scientific Officer for the All of Us Research Program. H.A.-C. and L.O.-M. were funded by NIH All of Us California grant (paid to her institute) (OT2OD026552). H.A.-C. also received NIH and Lon V. Smith Foundation funding outside this project. E.W.K. was funded by NIH 1OT2OD026553 and 3OT2OD026553-01S3. E.W.K. also received funding outside this work (2U01HG008685-05, 1OT2HL161841-01, P30AR070253, 1R01HL153805-01A1, R01AR063759-06A1, P30AR069625, R21AR078339). B.A.M. was funded by NIH U2OD023196. D.J.S. performed part of this work while a postdoctoral researcher at Vanderbilt University Medical Center and was funded by NIH grant 5 U2C OD023196-03. K.N.A. reports NIH grants (outside this work, paid to her institution) and consultancy with TrioHealth Inc and MedIQ (paid to her). K.N.A. is a consultant for the All of Us Research Program (paid to her). K.N.A. is a recipient of royalties from Cousera Specialization that is direct and a course that she teaches (payment made to her). K.A.G. reports Department of Defense and NIH grants (outside this work, paid to her institution) and consultancy with UptoDate, Teach for America, and Aspen Institute (paid to her), and Dr Gebo did this work when she was the Chief Medical and Scientific Officer for All of Us. A.R. was funded by All of Us Data and Research Center Grant, NIH OD (paid to her institute). A.R. also received funding outside this work from NIDDK UO1 Atypical Diabetes, PI (payment to her institute). B.M. was funded by NIH and DRC, and received NIH grants outside this work paid to his institute. D.B.G. is the CEO of Actio-Biosciences and co-founder and equity holder of Praxis-Precision Medicine and declares no conflicts of interests with this project. S.G. received support for this project from NIH (5U2COD023196-04). Q.C. reports funding from NIH outside this work paid to her institution. All other authors declare no competing interests.
Figures

References
-
- US Food and Drug Administration. 2020. EUA authorized serology test performance. US Food and Drug Administration, Silver Spring, MD. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-em....
-
- Pinto LA, Shawar RM, O’Leary B, Kemp TJ, Cherry J, Thornburg N, Miller CN, Gallagher PS, Stenzel T, Schuck B, Owen SM, Kondratovich M, Satheshkumar PS, Schuh A, Lester S, Cassetti MC, Sharpless NE, Gitterman S, Lowy DR. 2022. A trans-governmental collaboration to independently evaluate SARS-CoV-2 serology assays. Microbiol Spectr 10(1):e01564-21. doi:10.1128/spectrum.01564-21. - DOI - PMC - PubMed
-
- Guo L, Ren L, Yang S, Xiao M, Chang D, Yang F, Dela Cruz CS, Wang Y, Wu C, Xiao Y, Zhang L, Han L, Dang S, Xu Y, Yang Q-W, Xu S-Y, Zhu H-D, Xu Y-C, Jin Q, Sharma L, Wang L, Wang J. 2020. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin Infect Dis 71:778–785. doi:10.1093/cid/ciaa310. - DOI - PMC - PubMed
-
- Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, Wang X, Yuan J, Li T, Li J, Qian S, Hong C, Wang F, Liu Y, Wang Z, He Q, Li Z, He B, Zhang T, Fu Y, Ge S, Liu L, Zhang J, Xia N, Zhang Z. 2020. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin Infect Dis 71:2027–2034. doi:10.1093/cid/ciaa344. - DOI - PMC - PubMed
-
- Sherina N, Piralla A, Du L, Wan H, Kumagai-Braesch M, Andréll J, Braesch-Andersen S, Cassaniti I, Percivalle E, Sarasini A, Bergami F, Di Martino R, Colaneri M, Vecchia M, Sambo M, Zuccaro V, Bruno R, Sachs M, Oggionni T, Meloni F, Abolhassani H, Bertoglio F, Schubert M, Byrne-Steele M, Han J, Hust M, Xue Y, Hammarström L, Baldanti F, Marcotte H, Pan-Hammarström Q. 2021. Persistence of SARS-CoV-2-specific B and T cell responses in convalescent COVID-19 patients 6–8 months after the infection. Med (N Y) 2:281–295.e4. doi:10.1016/j.medj.2021.02.001. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
