A Multicenter Randomized Trial Evaluating the Insulin-Only Configuration of the Bionic Pancreas in Adults with Type 1 Diabetes
- PMID: 36173236
- PMCID: PMC9634987
- DOI: 10.1089/dia.2022.0200
A Multicenter Randomized Trial Evaluating the Insulin-Only Configuration of the Bionic Pancreas in Adults with Type 1 Diabetes
Abstract
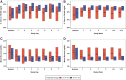
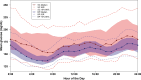
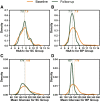
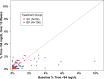
Objective: To evaluate the insulin-only configuration of the iLet® bionic pancreas (BP) using insulin aspart or insulin lispro in adults with type 1 diabetes (T1D). Methods: In this multicenter, randomized, controlled trial, 161 adults with T1D (18-79 years old, baseline HbA1c 5.5%-13.1%, 32% using multiple daily injections, 27% using a pump without automation, 5% using a pump with predictive low glucose suspend, and 36% using a hybrid closed loop system before the study) were randomly assigned 2:1 to use the BP (N = 107) with insulin aspart or insulin lispro (BP group) or a standard-of-care (SC) control group (N = 54) using their usual insulin delivery plus continuous glucose monitoring (CGM). The primary outcome was HbA1c at 13 weeks. Results: Mean HbA1c decreased from 7.6% ± 1.2% at baseline to 7.1% ± 0.6% at 13 weeks with BP versus 7.6% ± 1.2% to 7.5% ± 0.9% with SC (adjusted difference = -0.5%, 95% confidence interval -0.6% to -0.3%, P < 0.001). Over 13 weeks, mean time in range 70-180 mg/dL (TIR) increased by 11% (2.6 h/d) and mean CGM glucose was reduced by 16 mg/dL with BP compared with SC (P < 0.001). Improvement in these metrics was seen during the first day of BP use and by the end of the first week reached levels that remained relatively stable through 13 weeks. Analyses of time >180 mg/dL, time >250 mg/dL, and standard deviation of CGM glucose all favored the BP group (P < 0.001). The CGM-measured hypoglycemia was low at baseline (median time <54 mg/dL of 0.21% [3 min/d] for the BP group and 0.11% [1.6 min/d] for the SC group) and not significantly different between groups over the 13 weeks (P = 0.51 for time <70 mg/dL and 0.33 for time <54 mg/dL). There were 7 (6.5% of 107 participants) severe hypoglycemic events in the BP group and 2 events in the SC group (1.9% of 54 participants, P = 0.40). Conclusions: In adults with T1D, use of the BP with insulin aspart or insulin lispro improved HbA1c, TIR, and hyperglycemic metrics without increasing CGM-measured hypoglycemia compared with standard of care. Clinical Trial Registry: clinicaltrials.gov; NCT04200313.
Keywords: Adult; Artificial pancreas; Automated insulin delivery; Bionic pancreas; Evaluation; type 1 diabetes.
Conflict of interest statement
D.K. receives research funding from Abbott Diabetes Care, Dexcom, Inc., Novo Nordisk, and Beta Bionics, Inc.; advisory board funding from Abbott Diabetes Care, Novo Nordisk, Sanofi-aventis, Pendulum, and Medical Module; and speaker bureau from Dexcom, Inc., Novo Nordisk, Eli Lilly, Sanofi-aventis, BI Lilly, and Xeris. A.K. has no personal financial disclosures but reports that his employer's efforts were supported in part by grants from the NIH: UL1TR002489, P30DK124723. J.P. receives consulting fees from Sanofi, Novo Nordisk, Mannkind, Diasome, and Lilly and reports that his employer receives grant funding from Dexcom, Inc. and Eli Lilly. M.S. receives consulting fees from Eli Lilly and Neurocrine Biosciences and grant funding to his institution from Neurocrine Biosciences, Inc., and Mylan. S.T. receives research support from Insulet and Beta Bionics, Inc. S.J.R. has issued patents and pending patents on aspects of the BP that are assigned to Massachusetts General Hospital and licensed to Beta Bionics, Inc., has received honoraria and/or travel expenses for lectures from Novo Nordisk, Roche, and Ascensia Diabetes Care, serves on the scientific advisory boards of Unomedical, served on scientific advisory boards and had stock in Companion Medical that was bought out by Medtronic, has received consulting fees from Beta Bionics, Inc., Novo Nordisk, Senseonics, and Flexion Therapeutics, has received grant support from Zealand Pharma, Novo Nordisk, and Beta Bionics, Inc., and has received in-kind support in the form of technical support and/or donation of materials from Zealand Pharma, Ascencia, Senseonics, Adocia, and Tandem Diabetes. E.R.D. has issued patents and pending patents on aspects of the BP, and is an employee, the Executive Chair of the Board of Directors, and shareholder of Beta Bionics, Inc. F.H.E.-K. has issued patents and pending patents on aspects of the BP, and is an employee and shareholder of Beta Bionics, Inc. K.J.R. has no personal financial disclosures but reports that her employer has received grant support from Beta Bionics, Inc., Dexcom, Inc., and Tandem Diabetes Care. C.B. reports receiving consulting payments from Beta Bionics, Inc., Novo Nordisk, and Zealand Pharma. Z.L. has no personal financial disclosures but reports that her employer has received grant support from Beta Bionics, Inc., Dexcom, Inc., and Tandem Diabetes Care. M.C.M. has no personal financial disclosures but reports that his employer has received grant support from Beta Bionics, Inc., Dexcom, Inc., and Tandem Diabetes Care. P.C. is a former Dexcom, Inc. employee and his current employer has received consulting payments on his behalf from vTv Therapeutics, Beta Bionics, Inc., Dexcom, Inc., and Diasome. R.W.B. reports no personal financial disclosures but reports that his institution has received funding on his behalf as follows: grant funding and study supplies from Tandem Diabetes Care, Beta Bionics, Inc., and Dexcom, Inc.; study supplies from Medtronic, Ascencia, and Roche; consulting fees and study supplies from Eli Lilly and Novo Nordisk; and consulting fees from Insulet, Bigfoot Biomedical, vTv Therapeutics, and Diasome. All other authors have no personal financial disclosures to report.
Figures

References
-
- The Diabetes Control and Complications Trial Research Group: The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986. - PubMed
-
- American Diabetes Association Professional Practice Committee; Draznin B, Aroda VR, et al. : 6. Glycemic targets: standards of medical care in diabetes-2022. Diabetes Care 2022;45(Suppl 1):S83–S96. - PubMed
-
- Pettus JH, Zhou FL, Shepherd L, et al. : Incidences of severe hypoglycemia and diabetic ketoacidosis and prevalence of microvascular complications stratified by age and glycemic control in U.S. adult patients with type 1 diabetes: a real-world study. Diabetes Care 2019;42:2220–2227. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

