Design, Synthesis, and Characterization of Stapled Oligosaccharides
- PMID: 36173281
- PMCID: PMC9562281
- DOI: 10.1021/jacs.2c06882
Design, Synthesis, and Characterization of Stapled Oligosaccharides
Abstract
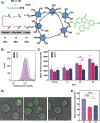
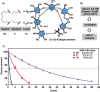
Stapling short peptides to lock specific conformations and thereby obtain superior pharmacological properties is well established. However, similar concepts have not been applied to oligosaccharides. Here, we describe the design, synthesis, and characterization of the first stapled oligosaccharides. Automated assembly of β-(1,6)-glucans equipped with two alkenyl side chains was followed by on-resin Grubbs metathesis for efficient ring closure with a variety of cross-linkers of different sizes. Oligosaccharide stapling increases enzymatic stability and cell penetration, therefore opening new opportunities for the use of glycans in medicinal chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Gellman S. H. Foldamers: A Manifesto. Acc. Chem. Res. 1998, 31, 173–180. 10.1021/ar960298r. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

