Treatment With Stereotactic Ablative Radiotherapy for Up to 5 Oligometastases in Patients With Cancer: Primary Toxic Effect Results of the Nonrandomized Phase 2 SABR-5 Clinical Trial
- PMID: 36173619
- PMCID: PMC9523552
- DOI: 10.1001/jamaoncol.2022.4394
Treatment With Stereotactic Ablative Radiotherapy for Up to 5 Oligometastases in Patients With Cancer: Primary Toxic Effect Results of the Nonrandomized Phase 2 SABR-5 Clinical Trial
Abstract
Importance: After the publication of the landmark SABR-COMET trial, concerns arose regarding high-grade toxic effects of treatment with stereotactic ablative body radiotherapy (SABR) for oligometastases.
Objective: To document toxic effects of treatment with SABR in a large cohort from a population-based, provincial cancer program.
Design, setting, and participants: From November 2016 to July 2020, 381 patients across all 6 cancer centers in British Columbia were treated in this single-arm, phase 2 trial of treatment with SABR for patients with oligometastatic or oligoprogressive disease. During this period, patients were only eligible to receive treatment with SABR in these settings in trials within British Columbia; therefore, this analysis is population based, with resultant minimal selection bias compared with previously published SABR series.
Interventions: Stereotactic ablative body radiotherapy to up to 5 metastases.
Main outcomes and measures: Rate of grade 2, 3, 4, and 5 toxic effects associated with SABR.
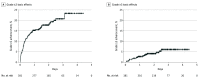
Findings: Among 381 participants (122 women [32%]), the mean (SD; range) age was 68 (11.1; 30-97) years, and the median (range) follow-up was 25 (1-54) months. The most common histological findings were prostate cancer (123 [32%]), colorectal cancer (63 [17%]), breast cancer (42 [11%]), and lung cancer (33 [9%]). The number of SABR-treated sites were 1 (263 [69%]), 2 (82 [22%]), and 3 or more (36 [10%]). The most common sites of SABR were lung (188 [34%]), nonspine bone (136 [25%]), spine (85 [16%]), lymph nodes (78 [14%]), liver (29 [5%]), and adrenal (15 [3%]). Rates of grade 2, 3, 4, and 5 toxic effects associated with SABR (based on the highest-grade toxic effect per patient) were 14.2%; (95% CI, 10.7%-17.7%), 4.2% (95% CI, 2.2%-6.2%), 0%, and 0.3% (95% CI, 0%-0.8%), respectively. The cumulative incidence of grade 2 or higher toxic effects associated with SABR at year 2 by Kaplan-Meier analysis was 8%, and for grade 3 or higher, 4%.
Conclusions and relevance: This single-arm, phase 2 clinical trial found that the incidence of grade 3 or higher SABR toxic effects in this population-based study was less than 5%. Furthermore, the rates of grade 2 or higher toxic effects (18.6%) were lower than previously published for SABR-COMET (29%). These results suggest that SABR treatment for oligometastases has acceptable rates of toxic effects and potentially support further enrollment in randomized phase 3 clinical trials.
Trial registration: ClinicalTrials.gov Identifier: NCT02933242.
Conflict of interest statement
Figures
Comment in
-
Defining a Therapeutic Ratio for Stereotactic Ablative Radiation Therapy in Oligometastatic Disease-Another Piece of the Puzzle.JAMA Oncol. 2022 Nov 1;8(11):1650-1651. doi: 10.1001/jamaoncol.2022.4342. JAMA Oncol. 2022. PMID: 36173641 No abstract available.
References
-
- Olson R, Senan S, Harrow S, et al. . Quality of life outcomes after stereotactic ablative radiation therapy (SABR) versus standard of care treatments in the oligometastatic setting: a secondary analysis of the SABR-COMET randomized trial. Int J Radiat Oncol Biol Phys. 2019;105(5):943-947. doi:10.1016/j.ijrobp.2019.08.041 - DOI - PubMed

