Randomized Clinical Trials of Machine Learning Interventions in Health Care: A Systematic Review
- PMID: 36173632
- PMCID: PMC9523495
- DOI: 10.1001/jamanetworkopen.2022.33946
Randomized Clinical Trials of Machine Learning Interventions in Health Care: A Systematic Review
Abstract
Importance: Despite the potential of machine learning to improve multiple aspects of patient care, barriers to clinical adoption remain. Randomized clinical trials (RCTs) are often a prerequisite to large-scale clinical adoption of an intervention, and important questions remain regarding how machine learning interventions are being incorporated into clinical trials in health care.
Objective: To systematically examine the design, reporting standards, risk of bias, and inclusivity of RCTs for medical machine learning interventions.
Evidence review: In this systematic review, the Cochrane Library, Google Scholar, Ovid Embase, Ovid MEDLINE, PubMed, Scopus, and Web of Science Core Collection online databases were searched and citation chasing was done to find relevant articles published from the inception of each database to October 15, 2021. Search terms for machine learning, clinical decision-making, and RCTs were used. Exclusion criteria included implementation of a non-RCT design, absence of original data, and evaluation of nonclinical interventions. Data were extracted from published articles. Trial characteristics, including primary intervention, demographics, adherence to the CONSORT-AI reporting guideline, and Cochrane risk of bias were analyzed.
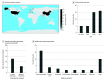
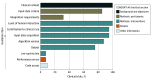
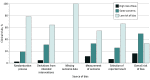
Findings: Literature search yielded 19 737 articles, of which 41 RCTs involved a median of 294 participants (range, 17-2488 participants). A total of 16 RCTS (39%) were published in 2021, 21 (51%) were conducted at single sites, and 15 (37%) involved endoscopy. No trials adhered to all CONSORT-AI standards. Common reasons for nonadherence were not assessing poor-quality or unavailable input data (38 trials [93%]), not analyzing performance errors (38 [93%]), and not including a statement regarding code or algorithm availability (37 [90%]). Overall risk of bias was high in 7 trials (17%). Of 11 trials (27%) that reported race and ethnicity data, the median proportion of participants from underrepresented minority groups was 21% (range, 0%-51%).
Conclusions and relevance: This systematic review found that despite the large number of medical machine learning-based algorithms in development, few RCTs for these technologies have been conducted. Among published RCTs, there was high variability in adherence to reporting standards and risk of bias and a lack of participants from underrepresented minority groups. These findings merit attention and should be considered in future RCT design and reporting.
Conflict of interest statement
Figures

References
-
- Yue W, Wang Z, Chen H, Payne A, Liu X. Machine learning with applications in breast cancer diagnosis and prognosis. Designs. 2018;2(2):13. doi:10.3390/designs2020013 - DOI

