Durable biochemical response and safety with oral octreotide capsules in acromegaly
- PMID: 36173649
- PMCID: PMC9641789
- DOI: 10.1530/EJE-22-0220
Durable biochemical response and safety with oral octreotide capsules in acromegaly
Abstract
Objective: The objective of this study is to report results from the open-label extension (OLE) of the OPTIMAL trial of oral octreotide capsules (OOC) in adults with acromegaly, evaluating the long-term durability of therapeutic response.
Design: The study design is an OLE of a double-blind placebo-controlled (DPC) trial.
Methods: Patients completing the 36-week DPC period on the study drug (OOC or placebo) or meeting predefined withdrawal criteria were eligible for OLE enrollment at 60 mg/day OOC dose, with the option to titrate to 40 or 80 mg/day. The OLE is ongoing; week 48 results are reported.
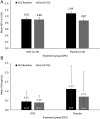
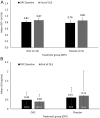
Results: Forty patients were enrolled in the OLE, 20 each having received OOC or placebo, with 14 and 5 patients completing the DPC period as responders, respectively. Ninety percent of patients completing the DPC period on OOC and 70% of those completing on placebo completed 48 weeks of the OLE. Maintenance of response in the OLE (i.e. insulin-like growth factor I (IGF1) ≤ 1.0 × upper limit of normal (ULN)) was achieved by 92.6% of patients who responded to OOC during the DPC period. Mean IGF1 levels were maintained between the end of the DPC period (0.91 × ULN; 95% CI: 0.784, 1.045) and week 48 of the OLE (0.90 × ULN; 95% CI: 0.750, 1.044) for those completing the DPC period on OOC. OOC safety was consistent with previous findings, with no increased adverse events (AEs) associated with the higher dose and improved gastrointestinal tolerability observed over time.
Conclusions: Patients with acromegaly maintained long-term biochemical response while receiving OOC, with no new AEs observed with prolonged OOC exposure.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

