A heterozygous LAMA5 variant may contribute to slowly progressive, vinculin-enhanced familial FSGS and pulmonary defects
- PMID: 36173685
- PMCID: PMC9746903
- DOI: 10.1172/jci.insight.158378
A heterozygous LAMA5 variant may contribute to slowly progressive, vinculin-enhanced familial FSGS and pulmonary defects
Abstract
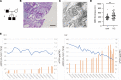
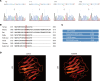
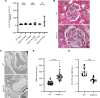
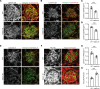
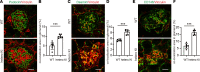
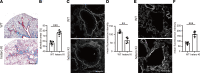
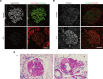
The LAMA5 gene encodes laminin α5, an indispensable component of glomerular basement membrane and other types of basement membrane. A homozygous pathological variant in LAMA5 is known to cause a systemic developmental syndrome including glomerulopathy. However, the roles of heterozygous LAMA5 gene variants in human renal and systemic diseases have remained unclear. We performed whole-exome sequencing analyses of a family with slowly progressive nephropathy associated with hereditary focal segmental glomerulosclerosis, and we identified what we believe to be a novel probable pathogenic variant of LAMA5, NP_005551.3:p.Val3687Met. In vitro analyses revealed cell type-dependent changes in secretion of variant laminin α5 laminin globular 4-5 (LG4-5) domain. Heterozygous and homozygous knockin mice with a corresponding variant of human LAMA5, p.Val3687Met, developed focal segmental glomerulosclerosis-like pathology with reduced laminin α5 and increased glomerular vinculin levels, which suggested that impaired cell adhesion may underlie this glomerulopathy. We also identified pulmonary defects such as bronchial deformity and alveolar dilation. Reexaminations of the family revealed phenotypes compatible with reduced laminin α5 and increased vinculin levels in affected tissues. Thus, the heterozygous p.Val3687Met variant may cause a new syndromic nephropathy with focal segmental glomerulosclerosis through possibly defective secretion of laminin α5. Enhanced vinculin may be a useful disease marker.
Keywords: Extracellular matrix; Genetic diseases; Genetics; Nephrology.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

