The choroid plexus: a missing link in our understanding of brain development and function
- PMID: 36173801
- PMCID: PMC9678431
- DOI: 10.1152/physrev.00060.2021
The choroid plexus: a missing link in our understanding of brain development and function
Abstract
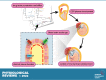
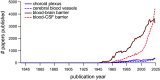
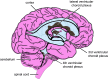
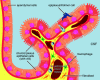
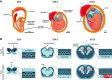
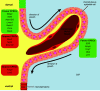
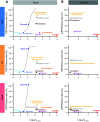
Studies of the choroid plexus lag behind those of the more widely known blood-brain barrier, despite a much longer history. This review has two overall aims. The first is to outline long-standing areas of research where there are unanswered questions, such as control of cerebrospinal fluid (CSF) secretion and blood flow. The second aim is to review research over the past 10 years where the focus has shifted to the idea that there are choroid plexuses located in each of the brain's ventricles that make specific contributions to brain development and function through molecules they generate for delivery via the CSF. These factors appear to be particularly important for aspects of normal brain growth. Most research carried out during the twentieth century dealt with the choroid plexus, a brain barrier interface making critical contributions to the composition and stability of the brain's internal environment throughout life. More recent research in the twenty-first century has shown the importance of choroid plexus-generated CSF in neurogenesis, influence of sex and other hormones on choroid plexus function, and choroid plexus involvement in circadian rhythms and sleep. The advancement of technologies to facilitate delivery of brain-specific therapies via the CSF to treat neurological disorders is a rapidly growing area of research. Conversely, understanding the basic mechanisms and implications of how maternal drug exposure during pregnancy impacts the developing brain represents another key area of research.
Keywords: blood-brain barrier; cerebrospinal fluid; choroid plexus; development; drug penetration.
Conflict of interest statement
S.A.L. maintains a financial interest in AstronauTx, Ltd., and is on the Scientific Advisory Board of RM Global.
Figures


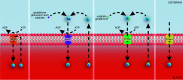
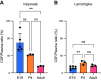
References
-
- Weed LH. The cerebrospinal fluid. Physiol Rev 2: 171–203, 1922. doi:10.1152/physrev.1922.2.2.171. - DOI
-
- Stern L, Gautier R. Recherches sur le liquide cephalo-rachidien. Arch Int Physiol 17: 138–192, 1921. doi:10.3109/13813452109146211. - DOI
-
- Stern L, Gautier R. Le passage dans le liquide cephalo-rachidien de substances introduites dans la circulation et leur action sur le systeme nerveux central chez les differentes especes animales. RCR Soc Phys Hist Nat Genève 35: 91–94, 1918.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

