Reaction of Thiosulfate Dehydrogenase with a Substrate Mimic Induces Dissociation of the Cysteine Heme Ligand Giving Insights into the Mechanism of Oxidative Catalysis
- PMID: 36173876
- PMCID: PMC9562282
- DOI: 10.1021/jacs.2c06062
Reaction of Thiosulfate Dehydrogenase with a Substrate Mimic Induces Dissociation of the Cysteine Heme Ligand Giving Insights into the Mechanism of Oxidative Catalysis
Abstract
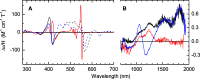
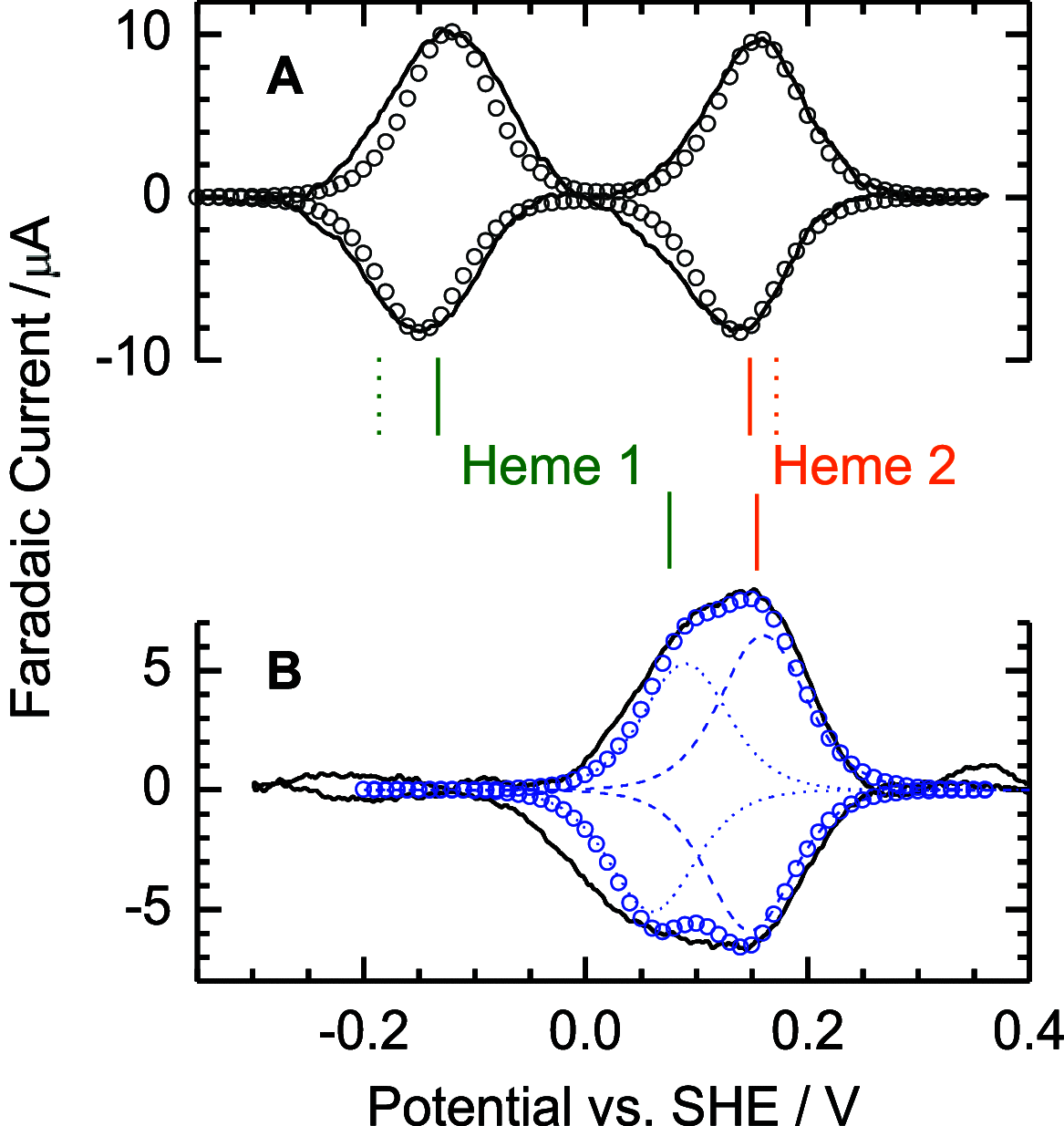
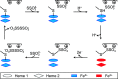
Thiosulfate dehydrogenases are bacterial cytochromes that contribute to the oxidation of inorganic sulfur. The active sites of these enzymes contain low-spin c-type heme with Cys-/His axial ligation. However, the reduction potentials of these hemes are several hundred mV more negative than that of the thiosulfate/tetrathionate couple (Em, +198 mV), making it difficult to rationalize the thiosulfate oxidizing capability. Here, we describe the reaction of Campylobacter jejuni thiosulfate dehydrogenase (TsdA) with sulfite, an analogue of thiosulfate. The reaction leads to stoichiometric conversion of the active site Cys to cysteinyl sulfonate (Cα-CH2-S-SO3-) such that the protein exists in a form closely resembling a proposed intermediate in the pathway for thiosulfate oxidation that carries a cysteinyl thiosulfate (Cα-CH2-S-SSO3-). The active site heme in the stable sulfonated protein displays an Em approximately 200 mV more positive than the Cys-/His-ligated state. This can explain the thiosulfate oxidizing activity of the enzyme and allows us to propose a catalytic mechanism for thiosulfate oxidation. Substrate-driven release of the Cys heme ligand allows that side chain to provide the site of substrate binding and redox transformation; the neighboring heme then simply provides a site for electron relay to an appropriate partner. This chemistry is distinct from that displayed by the Cys-ligated hemes found in gas-sensing hemoproteins and in enzymes such as the cytochromes P450. Thus, a further class of thiolate-ligated hemes is proposed, as exemplified by the TsdA centers that have evolved to catalyze the controlled redox transformations of inorganic oxo anions of sulfur.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Heme ligation and redox chemistry in two bacterial thiosulfate dehydrogenase (TsdA) enzymes.J Biol Chem. 2019 Nov 22;294(47):18002-18014. doi: 10.1074/jbc.RA119.010084. Epub 2019 Aug 29. J Biol Chem. 2019. PMID: 31467084 Free PMC article.
-
Mechanism of thiosulfate oxidation in the SoxA family of cysteine-ligated cytochromes.J Biol Chem. 2015 Apr 3;290(14):9209-21. doi: 10.1074/jbc.M114.618025. Epub 2015 Feb 11. J Biol Chem. 2015. PMID: 25673696 Free PMC article.
-
Thiosulfate dehydrogenase (TsdA) from Allochromatium vinosum: structural and functional insights into thiosulfate oxidation.J Biol Chem. 2015 Apr 3;290(14):9222-38. doi: 10.1074/jbc.M114.623397. Epub 2015 Feb 11. J Biol Chem. 2015. PMID: 25673691 Free PMC article.
-
The bacterial SoxAX cytochromes.Cell Mol Life Sci. 2013 Mar;70(6):977-92. doi: 10.1007/s00018-012-1098-y. Epub 2012 Aug 21. Cell Mol Life Sci. 2013. PMID: 22907414 Free PMC article. Review.
-
Oxidative metabolism of inorganic sulfur compounds by bacteria.Antonie Van Leeuwenhoek. 1997 Feb;71(1-2):95-107. doi: 10.1023/a:1000135707181. Antonie Van Leeuwenhoek. 1997. PMID: 9049021 Review.
Cited by
-
Extracellular catalysis of environmental substrates by Shewanella oneidensis MR-1 occurs via active sites on the C-terminal domains of MtrC.Protein Sci. 2025 Aug;34(8):e70243. doi: 10.1002/pro.70243. Protein Sci. 2025. PMID: 40725986 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

