Fast inference of spinal neuromodulation for motor control using amortized neural networks
- PMID: 36174534
- PMCID: PMC9668352
- DOI: 10.1088/1741-2552/ac9646
Fast inference of spinal neuromodulation for motor control using amortized neural networks
Abstract
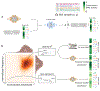
Objective.Epidural electrical stimulation (EES) has emerged as an approach to restore motor function following spinal cord injury (SCI). However, identifying optimal EES parameters presents a significant challenge due to the complex and stochastic nature of muscle control and the combinatorial explosion of possible parameter configurations. Here, we describe a machine-learning approach that leverages modern deep neural networks to learn bidirectional mappings between the space of permissible EES parameters and target motor outputs.Approach.We collected data from four sheep implanted with two 24-contact EES electrode arrays on the lumbosacral spinal cord. Muscle activity was recorded from four bilateral hindlimb electromyography (EMG) sensors. We introduce a general learning framework to identify EES parameters capable of generating desired patterns of EMG activity. Specifically, we first amortize spinal sensorimotor computations in a forward neural network model that learns to predict motor outputs based on EES parameters. Then, we employ a second neural network as an inverse model, which reuses the amortized knowledge learned by the forward model to guide the selection of EES parameters.Main results.We found that neural networks can functionally approximate spinal sensorimotor computations by accurately predicting EMG outputs based on EES parameters. The generalization capability of the forward model critically benefited our inverse model. We successfully identified novel EES parameters, in under 20 min, capable of producing desired target EMG recruitment duringin vivotesting. Furthermore, we discovered potential functional redundancies within the spinal sensorimotor networks by identifying unique EES parameters that result in similar motor outcomes. Together, these results suggest that our framework is well-suited to probe spinal circuitry and control muscle recruitment in a completely data-driven manner.Significance.We successfully identify novel EES parameters within minutes, capable of producing desired EMG recruitment. Our approach is data-driven, subject-agnostic, automated, and orders of magnitude faster than manual approaches.
Keywords: approximate Bayesian inference; artificial neural networks; epidural electrical stimulation; machine learning; neuromodulation; spinal cord stimulation.
© 2022 IOP Publishing Ltd.
Conflict of interest statement
Figures


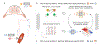



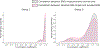

References
-
- Chauhan Neelima B. Chronic neurodegenerative consequences of traumatic brain injury. Restorative neurology and neuroscience, 32(2):337–365, 2014. - PubMed
-
- Courtine Grégoire and Sofroniew Michael V. Spinal cord repair: advances in biology and technology. Nature medicine, 25(6):898–908, 2019. - PubMed
-
- James Nicholas D, McMahon Stephen B, Field-Fote Edelle C, and Bradbury Elizabeth J. Neuromodulation in the restoration of function after spinal cord injury. The Lancet Neurology, 17(10):905–917, 2018. ISSN 14744422. doi: 10.1016/S1474-4422(18)30287-4. URL https://linkinghub.elsevier.com/retrieve/pii/S1474442218302874. - DOI - PubMed
-
- Calvert Jonathan S, Grahn Peter J, Strommen Jeffrey A, Lavrov Igor A, Beck Lisa A, Gill Megan L, Linde Margaux B, Brown Desmond A, Van Straaten Meegan G, Veith Daniel D, et al. Electrophysiological guidance of epidural electrode array implantation over the human lumbosacral spinal cord to enable motor function after chronic paralysis. Journal of neurotrauma, 36(9):1451–1460, 2019. - PMC - PubMed
-
- Gill Megan L, Grahn Peter J, Calvert Jonathan S, Linde Margaux B, Lavrov Igor A, Strommen Jeffrey A, Beck Lisa A, Sayenko Dimitry G, Van Straaten Meegan G, Drubach Dina I, et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nature medicine, 24(11):1677–1682, 2018. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
