Bioelectronic medicine: Preclinical insights and clinical advances
- PMID: 36174571
- PMCID: PMC10155266
- DOI: 10.1016/j.neuron.2022.09.003
Bioelectronic medicine: Preclinical insights and clinical advances
Abstract
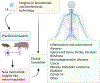
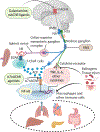
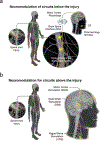
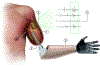
The nervous system maintains homeostasis and health. Homeostatic disruptions underlying the pathobiology of many diseases can be controlled by bioelectronic devices targeting CNS and peripheral neural circuits. New insights into the regulatory functions of the nervous system and technological developments in bioelectronics drive progress in the emerging field of bioelectronic medicine. Here, we provide an overview of key aspects of preclinical research, translation, and clinical advances in bioelectronic medicine.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests K.J.T. and V.A.P. have co-authored patents broadly related to the content of this review. They have assigned their rights to the Feinstein Institutes for Medical Research. K.J.T. also declares that he is a consultant to Setpoint Medical.
Figures


References
-
- AL-ALY Z, XIE Y. & BOWE B. 2021. High-dimensional characterization of post-acute sequelae of COVID-19. Nature, 594, 259–264. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

