Epithelial Autoantigen-Specific IgG Antibody Enhances Eosinophil Extracellular Trap Formation in Severe Asthma
- PMID: 36174991
- PMCID: PMC9523418
- DOI: 10.4168/aair.2022.14.5.479
Epithelial Autoantigen-Specific IgG Antibody Enhances Eosinophil Extracellular Trap Formation in Severe Asthma
Abstract
Purpose: There have been autoimmune mechanisms for the pathogenesis of severe asthma (SA) involving epithelial autoantigen-specific antibodies. This study aimed to find the function of these antibodies in the formation of eosinophil extracellular traps (EETs), contributing to the development of SA.
Methods: Patients with SA (n = 11), those with patients with nonsevere asthma (NSA, n = 41), and healthy controls (HCs, n = 26) were recruited to evaluate levels of epithelial antigens and autoantigen-specific antibodies. Moreover, the significance of epithelial autoantigen-specific antibodies in association with EET production was investigated ex vivo and in vivo.
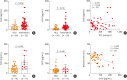
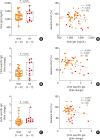
Results: Significantly higher levels of serum cytokeratin (CK) 18 and CK18-specific IgG were observed in patients with SA than in those with NSA (P = 0.001 and P = 0.031, respectively), while no differences were found in serum CK19 or CK19-specific immunoglobulin G (IgG). Moreover, levels of serum CK18 were positively correlated with total eosinophil counts (r = 0.276, P = 0.048) in asthmatics, while a negative correlation was noted between levels of serum CK18 and forced expiratory volume in 1 second (FEV1) %. In the presence of CK18-specific IgG, peripheral eosinophils from asthmatics released EETs, which further increased CK18 production from airway epithelial cells. In severe asthmatic mice, CK18 expression and CK18-specific IgG production were enhanced in the lungs, where EET treatment enhanced CK18 expression and CK18-specific IgG production, either of which was not suppressed by dexamethasone.
Conclusions: These suggest that EETs could enhance epithelial autoantigen (CK18)-induced autoimmune responses, further stimulating EET production and type 2 airway responses, which is a new therapeutic target for SA.
Keywords: Asthma; autoantigens; autoimmunity; eosinophils; epithelium; extracellular traps; immunoglobulin G; keratin.
Copyright © 2022 The Korean Academy of Asthma, Allergy and Clinical Immunology • The Korean Academy of Pediatric Allergy and Respiratory Disease.
Conflict of interest statement
There are no financial or other issues that might lead to conflict of interest.
Figures



References
-
- Cevhertas L, Ogulur I, Maurer DJ, Burla D, Ding M, Jansen K, et al. Advances and recent developments in asthma in 2020. Allergy. 2020;75:3124–3146. - PubMed
-
- Holguin F, Cardet JC, Chung KF, Diver S, Ferreira DS, Fitzpatrick A, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2020;55:1900588. - PubMed
-
- Soma T, Iemura H, Naito E, Miyauchi S, Uchida Y, Nakagome K, et al. Implication of fraction of exhaled nitric oxide and blood eosinophil count in severe asthma. Allergol Int. 2018;67S:S3–11. - PubMed

