Migration is not the perfect answer: How the cross-talk error correction for multiple breath nitrogen washout (MBWN2 ) parameters differs on directly collected vs. legacy data
- PMID: 36175005
- PMCID: PMC10092713
- DOI: 10.1002/ppul.26169
Migration is not the perfect answer: How the cross-talk error correction for multiple breath nitrogen washout (MBWN2 ) parameters differs on directly collected vs. legacy data
Abstract
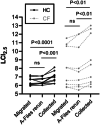
Recently, a cross-talk error with commercial multiple breath nitrogen washout (MBWN2 ) software was discovered, which produced an absolute over-reading of N2 of approximately 1%, i.e., 2% N2 read as 3%. This caused an extended tail to the washout, and over-estimated lung clearance index (LCI2.5 ) values. Subsequently an updated and corrected software version has been released. Within the field there have been discussions on how to correct legacy data, whether to migrate or completely "rerun" raw data A-files from the old software into the new corrected software. To our knowledge, no research has been published assessing whether either method is equivalent to directly collecting data in the new corrected software. We prospectively recruited 19 participants, 10 adult healthy controls and 9 people with cystic fibrosis (CF). MBWN2 was performed using the Exhalyzer® D first on the old 3.1.6 software and next, directly on corrected 3.3.1 software. Multiple breath washout (MBW) data directly collected in 3.3.1 was significantly different from both migrated and rerun data. A total of 7 of the 19 participants (37%; 4 CF) had a relative difference in LCI2.5 > 10% for both migrated and rerun data compared to 3.3.1 collected data. Our findings have implications for the Global Lung Initiative MBW project, which is accepting a combination of directly collected, A-file reruns and migrated data to establish normative values. Further, caution must be used in clinical practice when comparing corrected legacy data versus 3.3.1 collected data for clinical interpretation. We recommend that a new baseline is collected directly on 3.3.1. before clinical interpretation and decisions are determined when comparing consecutive MBW tests.
Keywords: biomarkers; cystic fibrosis (CF); lung clearance index (LCI); lung function testing; multiple breath washout (MBW); pulmonary function testing (PFT); pulmonary physiology.
© 2022 The Authors. Pediatric Pulmonology published by Wiley Periodicals LLC.
Conflict of interest statement
M.A., S.P., and O.P. have no conflict of interest to declare. C.S. (Christopher Short) and C.S. (Clare Saunders) have received speaker fees from Vertex pharmaceuticals. J.C.D. has performed clinical trial leadership roles, educational and/or advisory activities for the following: Abbvie, Algipharma AS, Bayer AG, Boehringer Ingelheim Pharma GmbH & Co. KG, Eloxx, Enterprise, Galapagos NV, Genentech, ImevaX GmbH, Ionis, LifeArc, Nivalis Therapeutics, Inc., Krystal Biotech, Novartis, PARI Medical Holding GmbH, ProQR Therapeutics III B.V., Proteostasis Therapeutics INC., Pulmocide, Raptor Pharmaceuticals Inc., Recode, Vertex Pharmaceuticals.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

