Developmentally regulated alternate 3' end cleavage of nascent transcripts controls dynamic changes in protein expression in an adult stem cell lineage
- PMID: 36175033
- PMCID: PMC9575692
- DOI: 10.1101/gad.349689.122
Developmentally regulated alternate 3' end cleavage of nascent transcripts controls dynamic changes in protein expression in an adult stem cell lineage
Abstract
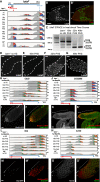
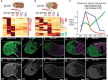
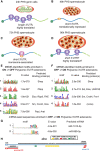
Alternative polyadenylation (APA) generates transcript isoforms that differ in the position of the 3' cleavage site, resulting in the production of mRNA isoforms with different length 3' UTRs. Although widespread, the role of APA in the biology of cells, tissues, and organisms has been controversial. We identified >500 Drosophila genes that express mRNA isoforms with a long 3' UTR in proliferating spermatogonia but a short 3' UTR in differentiating spermatocytes due to APA. We show that the stage-specific choice of the 3' end cleavage site can be regulated by the arrangement of a canonical polyadenylation signal (PAS) near the distal cleavage site but a variant or no recognizable PAS near the proximal cleavage site. The emergence of transcripts with shorter 3' UTRs in differentiating cells correlated with changes in expression of the encoded proteins, either from off in spermatogonia to on in spermatocytes or vice versa. Polysome gradient fractionation revealed >250 genes where the long 3' UTR versus short 3' UTR mRNA isoforms migrated differently, consistent with dramatic stage-specific changes in translation state. Thus, the developmentally regulated choice of an alternative site at which to make the 3' end cut that terminates nascent transcripts can profoundly affect the suite of proteins expressed as cells advance through sequential steps in a differentiation lineage.
Keywords: alternative polyadenylation; mRNA isoforms; spermatogenesis; translational control.
© 2022 Berry et al.; Published by Cold Spring Harbor Laboratory Press.
Figures



Similar articles
-
A developmental mechanism to regulate alternative polyadenylation in an adult stem cell lineage.Genes Dev. 2024 Aug 20;38(13-14):655-674. doi: 10.1101/gad.351649.124. Genes Dev. 2024. PMID: 39111825 Free PMC article.
-
A Developmental Mechanism to Regulate Alternative Polyadenylation in an Adult Stem Cell Lineage.bioRxiv [Preprint]. 2024 Jul 10:2024.03.18.585561. doi: 10.1101/2024.03.18.585561. bioRxiv. 2024. Update in: Genes Dev. 2024 Aug 20;38(13-14):655-674. doi: 10.1101/gad.351649.124. PMID: 38562704 Free PMC article. Updated. Preprint.
-
Regulation and function of alternative polyadenylation in development and differentiation.RNA Biol. 2023 Jan;20(1):908-925. doi: 10.1080/15476286.2023.2275109. Epub 2023 Oct 31. RNA Biol. 2023. PMID: 37906624 Free PMC article. Review.
-
Alternative 3' UTRs act as scaffolds to regulate membrane protein localization.Nature. 2015 Jun 18;522(7556):363-7. doi: 10.1038/nature14321. Epub 2015 Apr 20. Nature. 2015. PMID: 25896326 Free PMC article.
-
Sequential Polyadenylation to Enable Alternative mRNA 3' End Formation.Mol Cells. 2023 Jan 31;46(1):57-64. doi: 10.14348/molcells.2023.2176. Epub 2023 Jan 3. Mol Cells. 2023. PMID: 36697238 Free PMC article. Review.
Cited by
-
A developmental mechanism to regulate alternative polyadenylation in an adult stem cell lineage.Genes Dev. 2024 Aug 20;38(13-14):655-674. doi: 10.1101/gad.351649.124. Genes Dev. 2024. PMID: 39111825 Free PMC article.
-
Alternative cleavage and polyadenylation of the Ccnb1 mRNA defines accumulation of cyclin protein during the meiotic cell cycle.Nucleic Acids Res. 2024 Feb 9;52(3):1258-1271. doi: 10.1093/nar/gkad1151. Nucleic Acids Res. 2024. PMID: 38048302 Free PMC article.
-
A Developmental Mechanism to Regulate Alternative Polyadenylation in an Adult Stem Cell Lineage.bioRxiv [Preprint]. 2024 Jul 10:2024.03.18.585561. doi: 10.1101/2024.03.18.585561. bioRxiv. 2024. Update in: Genes Dev. 2024 Aug 20;38(13-14):655-674. doi: 10.1101/gad.351649.124. PMID: 38562704 Free PMC article. Updated. Preprint.
-
Cell-type-specific interacting proteins collaborate to regulate the timing of Cyclin B protein expression in male meiotic prophase.Development. 2023 Nov 15;150(22):dev201709. doi: 10.1242/dev.201709. Epub 2023 Nov 23. Development. 2023. PMID: 37882771 Free PMC article.
-
Regulation and function of alternative polyadenylation in development and differentiation.RNA Biol. 2023 Jan;20(1):908-925. doi: 10.1080/15476286.2023.2275109. Epub 2023 Oct 31. RNA Biol. 2023. PMID: 37906624 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
