Biallelic variants in the SLC13A1 sulfate transporter gene cause hyposulfatemia with a mild spondylo-epi-metaphyseal dysplasia
- PMID: 36175384
- PMCID: PMC10092256
- DOI: 10.1111/cge.14239
Biallelic variants in the SLC13A1 sulfate transporter gene cause hyposulfatemia with a mild spondylo-epi-metaphyseal dysplasia
Abstract
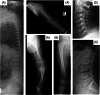
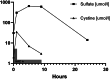
Sulfate is the fourth most abundant anion in human plasma but is not measured in clinical practice and little is known about the consequences of sulfate deficiency. Nevertheless, sulfation plays an essential role in the modulation of numerous compounds, including proteoglycans and steroids. We report the first patient with a homozygous loss-of-function variant in the SLC13A1 gene, encoding a renal and intestinal sulfate transporter, which is essential for maintaining plasma sulfate levels. The homozygous (Arg12Ter) variant in SLC13A1 was found by exome sequencing performed in a patient with unexplained skeletal dysplasia. The main clinical features were enlargement of joints and spondylo-epi-metaphyseal radiological abnormalities in early childhood, which improved with age. In addition, autistic features were noted. We found profound hyposulfatemia due to complete loss of renal sulfate reabsorption. Cholesterol sulfate was reduced. Intravenous N-acetylcysteine administration temporarily restored plasma sulfate levels. We conclude that loss of the SLC13A1 gene leads to profound hypersulfaturia and hyposulfatemia, which is mainly associated with abnormal skeletal development, possibly predisposing to degenerative bone and joint disease. The diagnosis might be easily missed and more frequent.
Keywords: acetylcysteine; autistic disorder; cholesterol; chondroitin; joint diseases; proteoglycans; skeletal dysplasia; sulfation.
© 2022 The Authors. Clinical Genetics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Biallelic SLC13A1 loss-of-function variants result in impaired sulfate transport and skeletal phenotypes, including short stature, scoliosis, and skeletal dysplasia.Genet Med Open. 2024 Dec 26;3:101958. doi: 10.1016/j.gimo.2024.101958. eCollection 2025. Genet Med Open. 2024. PMID: 39925707 Free PMC article.
-
SLC26A1 is a major determinant of sulfate homeostasis in humans.J Clin Invest. 2023 Feb 1;133(3):e161849. doi: 10.1172/JCI161849. J Clin Invest. 2023. PMID: 36719378 Free PMC article.
-
Slc13a1 and Slc26a1 KO models reveal physiological roles of anion transporters.Physiology (Bethesda). 2012 Feb;27(1):7-14. doi: 10.1152/physiol.00041.2011. Physiology (Bethesda). 2012. PMID: 22311966 Review.
-
Physiological roles of mammalian sulfate transporters NaS1 and Sat1.Arch Immunol Ther Exp (Warsz). 2011 Apr;59(2):113-6. doi: 10.1007/s00005-011-0114-5. Epub 2011 Feb 6. Arch Immunol Ther Exp (Warsz). 2011. PMID: 21298488 Review.
-
Partial deletion of the sulfate transporter SLC13A1 is associated with an osteochondrodysplasia in the Miniature Poodle breed.PLoS One. 2012;7(12):e51917. doi: 10.1371/journal.pone.0051917. Epub 2012 Dec 26. PLoS One. 2012. PMID: 23300579 Free PMC article.
Cited by
-
Coupling metabolomics and exome sequencing reveals graded effects of rare damaging heterozygous variants on gene function and human traits.Nat Genet. 2025 Jan;57(1):193-205. doi: 10.1038/s41588-024-01965-7. Epub 2025 Jan 2. Nat Genet. 2025. PMID: 39747595 Free PMC article.
-
Sulfur-Element containing metabolic pathways in human health and crosstalk with the microbiome.Biochem Biophys Rep. 2023 Aug 13;35:101529. doi: 10.1016/j.bbrep.2023.101529. eCollection 2023 Sep. Biochem Biophys Rep. 2023. PMID: 37601447 Free PMC article. Review.
-
Genetic determinants of urine and plasma metabolite levels.Nat Rev Nephrol. 2025 Jul;21(7):441-442. doi: 10.1038/s41581-025-00953-2. Nat Rev Nephrol. 2025. PMID: 40140728 No abstract available.
-
Biallelic SLC13A1 loss-of-function variants result in impaired sulfate transport and skeletal phenotypes, including short stature, scoliosis, and skeletal dysplasia.Genet Med Open. 2024 Dec 26;3:101958. doi: 10.1016/j.gimo.2024.101958. eCollection 2025. Genet Med Open. 2024. PMID: 39925707 Free PMC article.
-
Analytical methods for quantitating sulfate in plasma and serum.Essays Biochem. 2024 Dec 4;68(4):383-389. doi: 10.1042/EBC20230092. Essays Biochem. 2024. PMID: 38699863 Free PMC article. Review.
References
-
- Dawson PA. Role of sulphate in development. Reproduction. 2013;146(3):R81‐R89. - PubMed
-
- Langford R, Hurrion E, Dawson PA. Genetics and pathophysiology of mammalian sulfate biology. J Genet Genomics. 2017;44(1):7‐20. - PubMed
-
- Cole DE, Evrovski J. Quantitation of sulfate and thiosulfate in clinical samples by ion chromatography. J Chromatogr A. 1997;789(1–2):221‐232. - PubMed
-
- Lee A, Beck L, Markovich D. The human renal sodium sulfate cotransporter (SLC13A1; hNaSi‐1) cDNA and gene: organization, chromosomal localization, and functional characterization. Genomics. 2000;70(3):354‐363. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

