Visualizing germination of microbiota endospores in the mammalian gut
- PMID: 36175402
- PMCID: PMC9543051
- DOI: 10.1080/19490976.2022.2125737
Visualizing germination of microbiota endospores in the mammalian gut
Abstract
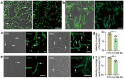
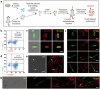
Transmission of bacterial endospores between the environment and people and the following germination in vivo play critical roles in both the deadly infections of some bacterial pathogens and the stabilization of the commensal microbiotas in humans. Our knowledge about the germination process of different bacteria in the mammalian gut, however, is still very limited due to the lack of suitable tools to visually monitor this process. We proposed a two-step labeling strategy that can image and quantify the endospores' germination in the recipient's intestines. Endospores collected from donor's gut microbiota were first labeled with fluorescein isothiocyanate and transplanted to mice via gavage. The recipient mice were then administered with Cyanine5-tagged D-amino acid to label all the viable bacteria, including the germinated endospores, in their intestines in situ. The germinated donor endospores could be distinguished by presenting two types of fluorescent signals simultaneously. The integrative use of cell-sorting, 16S rDNA sequencing, and fluorescence in situ hybridization (FISH) staining of the two-colored bacteria unveiled the taxonomic information of the donor endospores that germinated in the recipient's gut. Using this strategy, we investigated effects of different germinants and pre-treatment interventions on their germination, and found that germination of different commensal bacterial genera was distinctly affected by various types of germinants. This two-color labeling strategy shows its potential as a versatile tool for visually monitoring endospore germination in the hosts and screening for new interventions to improve endospore-based therapeutics.
Keywords: Bacterial endospore; FDAA; germination; in vivo metabolic labeling; two-color fluorescence imaging.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
