Identification of novel differentially expressed genes in type 1 diabetes mellitus complications using transcriptomic profiling of UAE patients: a multicenter study
- PMID: 36175575
- PMCID: PMC9523055
- DOI: 10.1038/s41598-022-18997-w
Identification of novel differentially expressed genes in type 1 diabetes mellitus complications using transcriptomic profiling of UAE patients: a multicenter study
Abstract
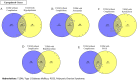
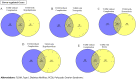
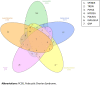
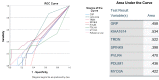
Type 1 diabetes mellitus (T1DM) is a chronic metabolic disorder that mainly affects children and young adults. It is associated with debilitating and long-life complications. Therefore, understanding the factors that lead to the onset and development of these complications is crucial. To our knowledge this is the first study that attempts to identify the common differentially expressed genes (DEGs) in T1DM complications using whole transcriptomic profiling in United Arab Emirates (UAE) patients. The present multicenter study was conducted in different hospitals in UAE including University Hospital Sharjah, Dubai Hospital and Rashid Hospital. A total of fifty-eight Emirati participants aged above 18 years and with a BMI < 25 kg/m2 were recruited and forty-five of these participants had a confirmed diagnosis of T1DM. Five groups of complications associated with the latter were identified including hyperlipidemia, neuropathy, ketoacidosis, hypothyroidism and polycystic ovary syndrome (PCOS). A comprehensive whole transcriptomic analysis using NGS was conducted. The outcomes of the study revealed the common DEGs between T1DM without complications and T1DM with different complications. The results revealed seven common candidate DEGs, SPINK9, TRDN, PVRL4, MYO3A, PDLIM1, KIAA1614 and GRP were upregulated in T1DM complications with significant increase in expression of SPINK9 (Fold change: 5.28, 3.79, 5.20, 3.79, 5.20) and MYO3A (Fold change: 4.14, 6.11, 2.60, 4.33, 4.49) in hyperlipidemia, neuropathy, ketoacidosis, hypothyroidism and PCOS, respectively. In addition, functional pathways of ion transport, mineral absorption and cytosolic calcium concentration were involved in regulation of candidate upregulated genes related to neuropathy, ketoacidosis and PCOS, respectively. The findings of this study represent a novel reference warranting further studies to shed light on the causative genetic factors that are involved in the onset and development of T1DM complications.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Comprehensive Analyses of Type 1 Diabetes Ketosis- or Ketoacidosis-Related Genes in Activated CD56+CD16+ NK Cells.Front Endocrinol (Lausanne). 2021 Nov 25;12:750135. doi: 10.3389/fendo.2021.750135. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34899600 Free PMC article.
-
Prevalence of Polycystic Ovary Syndrome amongst Females Aged between 15 and 45 Years at a Major Women's Hospital in Dubai, United Arab Emirates.Int J Environ Res Public Health. 2023 May 4;20(9):5717. doi: 10.3390/ijerph20095717. Int J Environ Res Public Health. 2023. PMID: 37174235 Free PMC article.
-
Serum anti-Müllerian hormone concentration in women with polycystic ovary syndrome and type 1 diabetes mellitus.Metabolism. 2016 May;65(5):804-811. doi: 10.1016/j.metabol.2016.02.005. Epub 2016 Feb 16. Metabolism. 2016. PMID: 26961579
-
Type 1 Diabetes Mellitus.Ann Intern Med. 2022 Mar;175(3):ITC33-ITC48. doi: 10.7326/AITC202203150. Epub 2022 Mar 8. Ann Intern Med. 2022. PMID: 35254878 Review.
-
Factors influencing the prevalence of polycystic ovary syndrome (PCOS) in the United Arab Emirates.Rev Environ Health. 2022 May 11;37(3):311-319. doi: 10.1515/reveh-2021-0036. Print 2022 Sep 27. Rev Environ Health. 2022. PMID: 35538690 Review.
Cited by
-
Developmental programming: Adipose depot-specific regulation of non-coding RNAs and their relation to coding RNA expression in prenatal testosterone and prenatal bisphenol-A -treated female sheep.Mol Cell Endocrinol. 2023 Mar 15;564:111868. doi: 10.1016/j.mce.2023.111868. Epub 2023 Jan 26. Mol Cell Endocrinol. 2023. PMID: 36708980 Free PMC article.
-
Protective Barriers Provided by the Epidermis.Int J Mol Sci. 2023 Feb 5;24(4):3145. doi: 10.3390/ijms24043145. Int J Mol Sci. 2023. PMID: 36834554 Free PMC article. Review.
References
-
- Monti MC, Lonsdale JT, Montomoli C, Montross R, Schlag E, Greenberg DA. Familial risk factors for microvascular complications and differential male-female risk in a large cohort of American families with type 1 diabetes. J. Clin. Endocrinol. Metab. 2007;92:4650–4655. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

