Philadelphia-negative myeloproliferative neoplasms display alterations in monocyte subpopulations frequency and immunophenotype
- PMID: 36175590
- PMCID: PMC9522456
- DOI: 10.1007/s12032-022-01825-6
Philadelphia-negative myeloproliferative neoplasms display alterations in monocyte subpopulations frequency and immunophenotype
Abstract
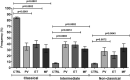
Philadelphia-negative myeloproliferative neoplasms (MPN) are clonal hematological diseases associated with driver mutations in JAK2, CALR, and MPL genes. Moreover, several evidence suggests that chronic inflammation and alterations in stromal and immune cells may contribute to MPN's pathophysiology. We evaluated the frequency and the immunophenotype of peripheral blood monocyte subpopulations in patients with polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (MF). Peripheral blood monocytes from PV (n = 16), ET (n = 16), and MF (n = 15) patients and healthy donors (n = 10) were isolated and submitted to immunophenotyping to determine the frequency of monocyte subpopulations and surface markers expression density. Plasma samples were used to measure the levels of soluble CD163, a biomarker of monocyte activity. PV, ET, and MF patients presented increased frequency of intermediate and non-classical monocytes and reduced frequency of classical monocytes compared to controls. Positivity for JAK2 mutation was significantly associated with the percentage of intermediate monocytes. PV, ET, and MF patients presented high-activated monocytes, evidenced by higher HLA-DR expression and increased soluble CD163 levels. The three MPN categories presented increased frequency of CD56+ aberrant monocytes, and PV and ET patients presented reduced frequency of CD80/86+ monocytes. Therefore, alterations in monocyte subpopulations frequency and surface markers expression pattern may contribute to oncoinflammation and may be associated with the pathophysiology of MPN.
Keywords: Flow cytometry; Immune cells; Inflammation; Monocytes; Myeloproliferative neoplasms; Pathophysiology.
© 2022. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures





References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

