Pharmacokinetics, efficacy and tolerance of cefoxitin in the treatment of cefoxitin-susceptible extended-spectrum beta-lactamase producing Enterobacterales infections in critically ill patients: a retrospective single-center study
- PMID: 36175707
- PMCID: PMC9522958
- DOI: 10.1186/s13613-022-01059-9
Pharmacokinetics, efficacy and tolerance of cefoxitin in the treatment of cefoxitin-susceptible extended-spectrum beta-lactamase producing Enterobacterales infections in critically ill patients: a retrospective single-center study
Abstract
Background: Cefoxitin is active against some extended-spectrum beta-lactamase-producing Enterobacterales (ESBL-PE), but has not been evaluated so far in the intensive care unit (ICU) settings. Data upon its pharmacokinetics (PK), tolerance and efficacy in critical conditions are scanty. We performed a retrospective single-center study in a university hospital medical ICU, in subjects presenting with cefoxitin-susceptible ESBL-PE infection and treated with cefoxitin. The primary aim was to determine cefoxitin PK. Secondary endpoints were efficacy, tolerance, and emergence of cephamycin-resistance.
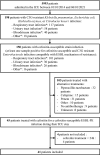
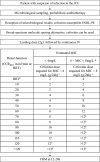
Results: Forty-one patients were included in this study, mainly with ESBL-PE pneumonia (35 patients, 85%). Cefoxitin was administered during a median [interquartile range (IQR)] duration of 5 [4-7] days. Cefoxitin serum concentrations strongly depended on renal function. Target serum concentration (> 5 × minimum inhibitory concentration (MIC) 24 h after cefoxitin onset was obtained in 34 patients (83%), using a median [IQR] daily dose of 6 [6-6] g with continuous administration. The standard dosage of 6 g/24 h was not sufficient to achieve the PK/PD target serum concentration for MIC up to 4-8 mg/L, except in patients with severe renal impairment and those treated with renal replacement therapy. Treatment failure occurred in 26 cases (63%), among whom 12 patients (29%) died, 13 patients (32%) were switched to alternative antibiotic therapy and 11 patients (27%) presented with relapse of infection with the same ESBL-PE. Serious adverse events attributed to cefoxitin occurred in 7 patients (17%). Acquisition of cephamycin-resistance with the same Enterobacterales was identified in 13 patients (32%), and was associated with underdosage.
Conclusion: Continuous administration of large doses of cefoxitin appears necessary to achieve the PK/PD target in patients with normal renal function. Renal status, MIC determination and therapeutic drug monitoring may be useful for treatment individualization in this setting. The treatment failure rate was 63%. The cefoxitin safety profile was favorable, but we observed a high rate of cephamycin-resistance emergence.
Keywords: Antibacterial chemotherapy; Carbapenem-sparing agents; Cefoxitin; Extended-spectrum beta-lactamase; Healthcare-associated pneumonia; Intensive care; Population pharmacokinetics.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- European Centre for Disease Prevention and Control (ECDC). Surveillance of antimicrobial resistance in Europe 2018. 2019.
-
- Detsis M, Karanika S, Mylonakis E. ICU acquisition rate, risk factors, and clinical significance of digestive tract colonization with extended-spectrum beta-lactamase-producing Enterobacteriaceae: a systematic review and meta-analysis. Crit Care Med. 2017;45(4):705–714. doi: 10.1097/CCM.0000000000002253. - DOI - PubMed
LinkOut - more resources
Full Text Sources

