Influence of management practice on the microbiota of a critically endangered species: a longitudinal study of kākāpō chick faeces and associated nest litter
- PMID: 36175950
- PMCID: PMC9523977
- DOI: 10.1186/s42523-022-00204-w
Influence of management practice on the microbiota of a critically endangered species: a longitudinal study of kākāpō chick faeces and associated nest litter
Abstract
Background: The critically endangered kākāpō is a flightless, nocturnal parrot endemic to Aotearoa New Zealand. Recent efforts to describe the gastrointestinal microbial community of this threatened herbivore revealed a low-diversity microbiota that is often dominated by Escherichia-Shigella bacteria. Given the importance of associated microbial communities to animal health, and increasing appreciation of their potential relevance to threatened species conservation, we sought to better understand the development of this unusual gut microbiota profile. To this end, we conducted a longitudinal analysis of faecal material collected from kākāpō chicks during the 2019 breeding season, in addition to associated nest litter material.
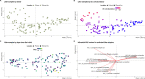
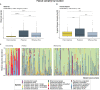
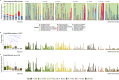
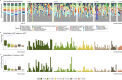
Results: Using an experimental approach rarely seen in studies of threatened species microbiota, we evaluated the impact of a regular conservation practice on the developing kākāpō microbiota, namely the removal of faecal material from nests. Artificially removing chick faeces from nests had negligible impact on bacterial community diversity for either chicks or nests (p > 0.05). However, the gut microbiota did change significantly over time as chick age increased (p < 0.01), with an increasing relative abundance of Escherichia-Shigella coli over the study period and similar observations for the associated nest litter microbiota (p < 0.01). Supplementary feeding substantially altered gut bacterial diversity of kākāpō chicks (p < 0.01), characterised by a significant increase in Lactobacillus bacteria.
Conclusions: Overall, chick age and hand rearing conditions had the most marked impact on faecal bacterial communities. Similarly, the surrounding nest litter microbiota changed significantly over time since a kākāpō chick was first placed in the nest, though we found no evidence that removal of faecal material influenced the bacterial communities of either litter or faecal samples. Taken together, these observations will inform ongoing conservation and management of this most enigmatic of bird species.
Keywords: Avian; Bird; Conservation; Experimental; Microbiome; Microbiota; Threatened.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Powlesland RG, Merton DV, Cockrem JF. A parrot apart: the natural history of the kakapo (Strigops habroptilus), and the context of its conservation management. Notornis. 2006;53:3–26.
-
- Merton DV, Morris RB, Atkinson IAE. Lek behaviour in a parrot: the kakapo Strigops habroptilus of New Zealand. Ibis. 1984;126:277–283. doi: 10.1111/j.1474-919x.1984.tb00250.x. - DOI
-
- White KL, Eason DK, Jamieson IG, Robertson BC. Evidence of inbreeding depression in the critically endangered parrot, the kakapo. Anim Conserv. 2015;18:341–347. doi: 10.1111/acv.12177. - DOI
-
- Savage JL, Crane JMS, Hemmings N. Low hatching success in the critically endangered kākāpō (Strigops habroptilus) is driven by early embryo mortality not infertility. Anim Conserv. 2021 doi: 10.1111/acv.12746. - DOI
