The prognostic value of early measures of the ventilatory ratio in the ARDS ROSE trial
- PMID: 36175982
- PMCID: PMC9521854
- DOI: 10.1186/s13054-022-04179-7
The prognostic value of early measures of the ventilatory ratio in the ARDS ROSE trial
Abstract
Background: The ventilatory ratio (VR, [minute ventilation × PaCO2]/[predicted body weight × 100 × 37.5]) is associated with mortality in ARDS. The aims of this study were to test whether baseline disease severity or neuromuscular blockade (NMB) modified the relationship between VR and mortality.
Methods: This was a post hoc analysis of the PETAL-ROSE trial, which randomized moderate-to-severe ARDS patients to NMB or control. Survival among patients with different VR trajectories or VR cutoff above and below the median was assessed by Kaplan-Meier analysis. The relationships between single-day or 48-h VR trajectories with 28- or 90-day mortality were tested by logistic regression. Randomization allocation to NMB and markers of disease severity were tested as confounders by multivariable regression and interaction term analyses.
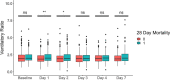
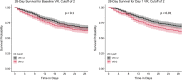
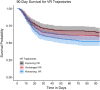
Results: Patients with worsening VR trajectories had significantly lower survival compared to those with improving VR (n = 602, p < 0.05). Patients with VR > 2 (median) at day 1 had a significantly lower 90-day survival compared to patients with VR ≤ 2 (HR 1.36, 95% CI 1.10-1.69). VR at day 1 was significantly associated with 28-day mortality (OR = 1.40, 95% CI 1.15-1.72). There was no interaction between NMB and VR for 28-day mortality. APACHE-III had a significant interaction with VR at baseline for the outcome of 28-day mortality, such that the relationship between VR and mortality was stronger among patients with lower APACHE-III. There was a significant association between rising VR trajectory and mortality that was independent of NMB, baseline PaO2/FiO2 ratio and generalized markers of disease severity (Adjusted OR 1.81, 95% CI 1.28-2.84 for 28-day and OR 2.07 95% CI 1.41-3.10 for 90-day mortality). APACHE-III and NMB were not effect modifiers in the relationship between VR trajectory and mortality.
Conclusions: Elevated baseline and day 1 VR were associated with higher 28-day mortality. The relationship between baseline VR and mortality was stronger among patients with lower APACHE-III. APACHE-III was not an effect modifier for the relationship between VR trajectory and mortality, so that the VR trajectory may be optimally suited for prognostication and predictive enrichment. VR was not different between patients randomized to NMB or control, indicating that VR can be utilized without correcting for NMB.
Keywords: APACHE-III; ARDS; Neuromuscular blockade; ROSE trial; VR; Ventilatory ratio.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

