Megaconial congenital muscular dystrophy due to novel CHKB variants: a case report and literature review
- PMID: 36175989
- PMCID: PMC9524117
- DOI: 10.1186/s13395-022-00306-8
Megaconial congenital muscular dystrophy due to novel CHKB variants: a case report and literature review
Abstract
Background: Choline kinase beta (CHKB) catalyzes the first step in the de novo biosynthesis of phosphatidyl choline and phosphatidylethanolamine via the Kennedy pathway. Derangement of this pathway might also influence the homeostasis of mitochondrial membranes. Autosomal recessive CHKB mutations cause a rare form of congenital muscular dystrophy known as megaconial congenital muscular dystrophy (MCMD).
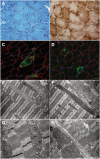
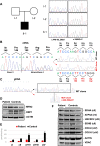
Case presentation: We describe a novel proband presenting MCMD due to unpublished CHKB mutations. The patient is a 6-year-old boy who came to our attention for cognitive impairment and slowly progressive muscular weakness. He was the first son of non-consanguineous healthy parents from Sri Lanka. Neurological examination showed proximal weakness at four limbs, weak osteotendinous reflexes, Gowers' maneuver, and waddling gate. Creatine kinase levels were mildly increased. EMG and brain MRI were normal. Left quadriceps skeletal muscle biopsy showed a myopathic pattern with nuclear centralizations and connective tissue increase. Histological and histochemical staining suggested subsarcolemmal localization and dimensional increase of mitochondria. Ultrastructural analysis confirmed the presence of enlarged ("megaconial") mitochondria. Direct sequencing of CHKB identified two novel defects: the c.1060G > C (p.Gly354Arg) substitution and the c.448-56_29del intronic deletion, segregating from father and mother, respectively. Subcloning of RT-PCR amplicons from patient's muscle RNA showed that c.448-56_29del results in the partial retention (14 nucleotides) of intron 3, altering physiological splicing and transcript stability. Biochemical studies showed reduced levels of the mitochondrial fission factor DRP1 and the severe impairment of mitochondrial respiratory chain activity in patient's muscle compared to controls.
Conclusions: This report expands the molecular findings associated with MCMD and confirms the importance of considering CHKB variants in the differential diagnosis of patients presenting with muscular dystrophy and mental retardation. The clinical outcome of MCMD patients seems to be influenced by CHKB molecular defects. Histological and ultrastructural examination of muscle biopsy directed molecular studies and allowed the identification and characterization of an intronic mutation, usually escaping standard molecular testing.
Keywords: Choline kinase beta (CHKB); Enlarged mitochondria; Megaconial congenital muscular dystrophy; Mitochondrial dynamics.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Mitsuhashi S, Ohkuma A, Talim B, Karahashi M, Koumura T, Aoyama C, Kurihara M, Quinlivan R, Sewry C, Mitsuhashi H, et al. A congenital muscular dystrophy with mitochondrial structural abnormalities caused by defective de novo phosphatidylcholine biosynthesis. Am J Hum Genet. 2011;88(6):845–851. doi: 10.1016/j.ajhg.2011.05.010. - DOI - PMC - PubMed
-
- Bardhan M, Polavarapu K, Bevinahalli NN, Veeramani PK, Anjanappa RM, Arunachal G, Shingavi L, Vengalil S, Nashi S, Chawla T, et al. NaMegaconial congenital muscular dystrophy secondary to novel CHKB mutations resemble atypical Rett syndrome. J Hum Genet. 2021;66(8):813–823. doi: 10.1038/s10038-021-00913-1. - DOI - PubMed
-
- Vanlander AV, Muiño Mosquera L, Panzer J, Deconinck T, Smet J, Seneca S, Van Dorpe J, Ferdinande L, Ceuterick-de Groote C, De Jonghe P, et al. Megaconial muscular dystrophy caused by mitochondrial membrane homeostasis defect, new insights from skeletal and heart muscle analyses. Mitochondrion. 2016;27:32–38. doi: 10.1016/j.mito.2016.02.001. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

