Design, synthesis and antitumor activity of 5-trifluoromethylpyrimidine derivatives as EGFR inhibitors
- PMID: 36176072
- PMCID: PMC9542405
- DOI: 10.1080/14756366.2022.2128797
Design, synthesis and antitumor activity of 5-trifluoromethylpyrimidine derivatives as EGFR inhibitors
Abstract
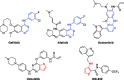
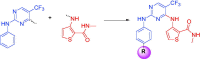
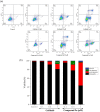
A new series of 5-trifluoromethylpyrimidine derivatives were designed and synthesised as EGFR inhibitors. Three tumour cells A549, MCF-7, PC-3 and EGFR kinase were employed to evaluate their biological activities. The results were shown that most of the target compounds existed excellent antitumor activities. In particular, the IC50 values of compound 9u (E)-3-((2-((4-(3-(3-fluorophenyl)acrylamido)phenyl)amino)-5-(trifluoromethyl)pyrimidin-4-yl)amino)-N-methylthiophene-2-carboxamide against A549, MCF-7, PC-3 cells and EGFR kinase reached to 0.35 μM, 3.24 μM, 5.12 μM, and 0.091 μM, respectively. Additionally, further researches revealed that compound 9u could induce early apoptosis of A549 cells and arrest the cells in G2/M phase. Taken together, these findings indicated that compound 9u was potential for developing as antitumor reagent.
Keywords: EGFR; antitumor; inhibitor; pyrimidine; reagent.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

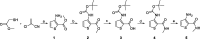




References
-
- Ayati A, Moghimi S, Salarinejad S, et al. A review on progression of epidermal growth factor receptor (EGFR) inhibitors as an efficient approach in cancer targeted therapy. Bioorg Chem. 2020;99:103811. - PubMed
-
- Yewale C, Baradia D, Vhora I, et al. Epidermal growth factor receptor targeting in cancer: a review of trends and strategies. Biomaterials. 2013;34(34):8690–707. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
