Multilayer CVD graphene electrodes using a transfer-free process for the next generation of optically transparent and MRI-compatible neural interfaces
- PMID: 36176270
- PMCID: PMC9512798
- DOI: 10.1038/s41378-022-00430-x
Multilayer CVD graphene electrodes using a transfer-free process for the next generation of optically transparent and MRI-compatible neural interfaces
Abstract
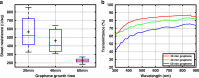
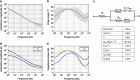
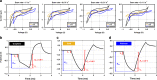
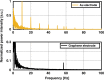
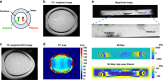
Multimodal platforms combining electrical neural recording and stimulation, optogenetics, optical imaging, and magnetic resonance (MRI) imaging are emerging as a promising platform to enhance the depth of characterization in neuroscientific research. Electrically conductive, optically transparent, and MRI-compatible electrodes can optimally combine all modalities. Graphene as a suitable electrode candidate material can be grown via chemical vapor deposition (CVD) processes and sandwiched between transparent biocompatible polymers. However, due to the high graphene growth temperature (≥ 900 °C) and the presence of polymers, fabrication is commonly based on a manual transfer process of pre-grown graphene sheets, which causes reliability issues. In this paper, we present CVD-based multilayer graphene electrodes fabricated using a wafer-scale transfer-free process for use in optically transparent and MRI-compatible neural interfaces. Our fabricated electrodes feature very low impedances which are comparable to those of noble metal electrodes of the same size and geometry. They also exhibit the highest charge storage capacity (CSC) reported to date among all previously fabricated CVD graphene electrodes. Our graphene electrodes did not reveal any photo-induced artifact during 10-Hz light pulse illumination. Additionally, we show here, for the first time, that CVD graphene electrodes do not cause any image artifact in a 3T MRI scanner. These results demonstrate that multilayer graphene electrodes are excellent candidates for the next generation of neural interfaces and can substitute the standard conventional metal electrodes. Our fabricated graphene electrodes enable multimodal neural recording, electrical and optogenetic stimulation, while allowing for optical imaging, as well as, artifact-free MRI studies.
Keywords: Engineering; Other nanotechnology.
© The Author(s) 2022.
Conflict of interest statement
Conflict of interestThe authors declare no competing interests.
Figures


References
LinkOut - more resources
Full Text Sources
