HOX genes in stem cells: Maintaining cellular identity and regulation of differentiation
- PMID: 36176275
- PMCID: PMC9514042
- DOI: 10.3389/fcell.2022.1002909
HOX genes in stem cells: Maintaining cellular identity and regulation of differentiation
Abstract
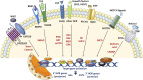
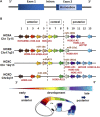
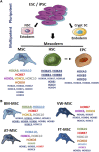
Stem cells display a unique cell type within the body that has the capacity to self-renew and differentiate into specialized cell types. Compared to pluripotent stem cells, adult stem cells (ASC) such as mesenchymal stem cells (MSCs) and hematopoietic stem cells (HSCs) exhibit restricted differentiation capabilities that are limited to cell types typically found in the tissue of origin, which implicates that there must be a certain code or priming determined by the tissue of origin. HOX genes, a subset of homeobox genes encoding transcription factors that are generally repressed in undifferentiated pluripotent stem cells, emerged here as master regulators of cell identity and cell fate during embryogenesis, and in maintaining this positional identity throughout life as well as specifying various regional properties of respective tissues. Concurrently, intricate molecular circuits regulated by diverse stem cell-typical signaling pathways, balance stem cell maintenance, proliferation and differentiation. However, it still needs to be unraveled how stem cell-related signaling pathways establish and regulate ASC-specific HOX expression pattern with different temporal-spatial topography, known as the HOX code. This comprehensive review therefore summarizes the current knowledge of specific ASC-related HOX expression patterns and how these were integrated into stem cell-related signaling pathways. Understanding the mechanism of HOX gene regulation in stem cells may provide new ways to manipulate stem cell fate and function leading to improved and new approaches in the field of regenerative medicine.
Keywords: HOX code; HSC; MSC; adult stem cell; differentiation; mesenchymal stem cell; signaling pathways.
Copyright © 2022 Steens and Klein.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

