B Cell Receptor Signaling Pathway Mutation as Prognosis Predictor of Immune Checkpoint Inhibitors in Lung Adenocarcinoma by Bioinformatic Analysis
- PMID: 36176353
- PMCID: PMC9514294
- DOI: 10.2147/JIR.S379016
B Cell Receptor Signaling Pathway Mutation as Prognosis Predictor of Immune Checkpoint Inhibitors in Lung Adenocarcinoma by Bioinformatic Analysis
Abstract
Purpose: The advent of immune checkpoint inhibitors (ICIs) is a revolutionary breakthrough. However, without the selection of a specific target population, the response rate of ICI therapy in lung adenocarcinoma (LUAD) is low, so a clinical challenge has arisen in effectively using biomarkers to determine which patients can benefit from ICI therapy.
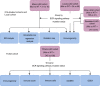
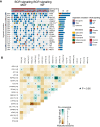
Methods: In this study, patients were divided according to whether or not nonsynonymous mutations were present in the BCR signaling pathway, and univariate and multivariate Cox regression models were established based on a LUAD cohort treated with ICIs (Miao-LUAD). Then the relationship between the mutation status of the BCR signaling pathway and the prognosis of immunotherapy was examined. Finally, data from The Cancer Genome Atlas (TCGA) LUAD cohort, the Rizvi-LUAD, the Samstein-LUAD, and the Zhujiang Hospital of Southern Medical University LUAD (Local-LUAD) cohort were combined, and the mutation panorama, immunogenicity, tumor microenvironment (TME) and pathway enrichment analysis between the BCR signaling pathway mutant group (BCR signaling MUT) and the BCR signaling pathway wild group (BCR signaling WT) were comprehensively compared.
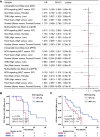
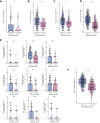
Results: It was found that, compared with the BCR signaling WT, the BCR signaling MUT had a significantly improved progression-free survival (PFS) rate and overall survival (OS) rate, higher immunogenicity (tumor mutational burden, neoantigen load, and DNA damage response signaling mutations), and anti-tumor immune microenvironment.
Conclusion: These results revealed that the mutation state of the BCR signaling pathway has potential as a biomarker to predict the efficacy of ICIs in LUAD.
Keywords: B cell receptor; biomarker; immune checkpoint inhibitor; microenvironment; non-small cell lung cancer.
© 2022 Lin et al.
Conflict of interest statement
The authors declare no conflicts of interest in relation to this work and that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

