Persistent or new symptoms 1 year after a single high dose of vitamin D3 in patients with moderate to severe COVID-19
- PMID: 36176639
- PMCID: PMC9513442
- DOI: 10.3389/fnut.2022.979667
Persistent or new symptoms 1 year after a single high dose of vitamin D3 in patients with moderate to severe COVID-19
Abstract
Purpose: The aim of this study was to investigate the reported persistent or new symptoms 1 year after a single dose of 200,000 IU of vitamin D3 and hospitalization in patients with moderate to severe COVID-19.
Methods: This is a post-hoc, exploratory analysis from a multicenter, double-blind, placebo-controlled, randomized clinical trial from two hospitals in São Paulo, Brazil, registered in ClinicalTrials.gov, NCT04449718. Discharged patients were followed for up to 1 year and evaluated by telephone interviews at 6 and 12 months. The primary and secondary outcomes were previously published. These post-hoc exploratory secondary outcomes are the persistent or new symptoms and quality of life (QoL) at the post-viral stage of COVID-19. Generalized estimating equations (GEE) for repeated measures with Bonferroni's adjustment were used for testing outcomes.
Results: Between 2 June and 27 August 2020, we randomized 240 patients of which 144 were included in this study [the vitamin D3 (n = 71) or placebo (n = 73) group]. The mean (SD) age was 54.3 (13.1) years, and body mass index (BMI) was 32.4 (6.5) kg/m2. Fever demonstrated a significant main effect of time (P < 0.001) with a reduction from baseline to 6 (52-0) and 12 months (52-0). No significant differences between groups were observed for fever, cough, fatigue, fever, myalgia, joint pain, runny nose, nasal congestion, sore throat, hypertension, diabetes, cardiovascular disease, rheumatic disease, asthma, chronic obstructive pulmonary, chronic kidney disease, QoL, and new or persistent symptoms up to 1-year of follow-up.
Conclusion: The findings do not support the use of 200,000 IU of vitamin D3 compared to placebo for the management of persistence or new symptoms, and QoL reported by moderate to severe patients after hospitalization for COVID-19.
Keywords: SARS-CoV-2; persistent symptoms; post-COVID-19; post-viral stage; quality of life; vitamin D.
Copyright © 2022 Fernandes, Sales, Santos, Caparbo, Murai and Pereira.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
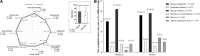

References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

