Role of extracellular vesicles secretion in paclitaxel resistance of prostate cancer cells
- PMID: 36176762
- PMCID: PMC9511801
- DOI: 10.20517/cdr.2022.26
Role of extracellular vesicles secretion in paclitaxel resistance of prostate cancer cells
Abstract
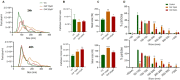
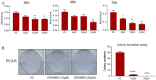
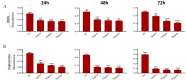
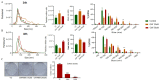
Aim: The development of chemotherapy resistance is the major obstacle in the treatment of advanced prostate cancer (PCa). Extracellular vesicles (EVs) secretion plays a significant role among different mechanisms contributing to chemoresistance. Hence, inhibition of EVs release may increase the efficacy of chemotherapeutic drugs against PCa. Methods: Paclitaxel (PTX) resistant PCa cells (PC3-R and DU145-R) were treated with GW4869, a known exosome biogenesis inhibitor. EVs were isolated from the conditioned media by ExoQuick-based precipitation method and characterized for concentration and size distribution by nanoparticle tracking analysis. The effect of GW4869 treatment on the survival and growth of PCa cells was assessed by MTT, and colony formation assays in vitro, and ectopic PC3-R xenografts in male athymic nude mice in vivo. The effect of other EV biogenesis inhibitors, imipramine and dimethyl amiloride (DMA), treatment was also analyzed on the survival of PC3-R cells. Results: GW4869 (10-20 µM) treatment of PTX resistant PCa cells significantly reduced the release of small EVs (50-100 nm size range) while increasing the release of larger EVs (> 150 nm in size), and inhibited their clonogenicity. Moreover, GW4869 (5-20 µM) treatment (24-72h) significantly inhibited the survival of PC3-R cells in a dose-dependent manner. We observed a similar growth inhibition with both imipramine (5-20 µg/mL) and DMA (5-20 µg/mL) treatment in PC3-R cells. Furthermore, GW4869 treatment (IP) in mice bearing PC3-R xenografts significantly reduced the tumor weight (65% reduction, P = 0.017) compared to the vehicle-treated control mice without causing any noticeable toxicity. Conclusion: Inhibiting the release of EVs could sensitize the resistant PCa cells to chemotherapy.
Keywords: GW4869; Prostate cancer; chemoresistance; extracellular vesicles; paclitaxel.
© The Author(s) 2022.
Conflict of interest statement
All authors declared that there are no conflicts of interest.
Figures

References
-
- Trachtenberg J. Hormonal management of stage D carcinoma of the prostate. Urol Clin North Am. 1987;14:685–94.. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
