Cooperative coordination-mediated multi-component self-assembly of "all-in-one" nanospike theranostic nano-platform for MRI-guided synergistic therapy against breast cancer
- PMID: 36176903
- PMCID: PMC9513557
- DOI: 10.1016/j.apsb.2022.02.027
Cooperative coordination-mediated multi-component self-assembly of "all-in-one" nanospike theranostic nano-platform for MRI-guided synergistic therapy against breast cancer
Abstract
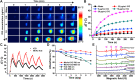
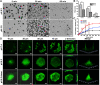
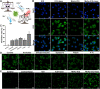
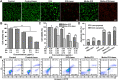
Carrier-free multi-component self-assembled nano-systems have attracted widespread attention owing to their easy preparation, high drug-loading efficiency, and excellent therapeutic efficacy. Herein, MnAs-ICG nanospike was generated by self-assembly of indocyanine green (ICG), manganese ions (Mn2+), and arsenate (AsO4 3-) based on electrostatic and coordination interactions, effectively integrating the bimodal imaging ability of magnetic resonance imaging (MRI) and fluorescence (FL) imaging-guided synergistic therapy of photothermal/chemo/chemodynamic therapy within an "all-in-one" theranostic nano-platform. The as-prepared MnAs-ICG nanospike had a uniform size, well-defined nanospike morphology, and impressive loading capacities. The MnAs-ICG nanospike exhibited sensitive responsiveness to the acidic tumor microenvironment with morphological transformation and dimensional variability, enabling deep penetration into tumor tissue and on-demand release of functional therapeutic components. In vitro and in vivo results revealed that MnAs-ICG nanospike showed synergistic tumor-killing effect, prolonged blood circulation and increased tumor accumulation compared to their individual components, effectively resulting in synergistic therapy of photothermal/chemo/chemodynamic therapy with excellent anti-tumor effect. Taken together, this new strategy might hold great promise for rationally engineering multifunctional theranostic nano-platforms for breast cancer treatment.
Keywords: Breast cancer; Carrier-free nanodrugs; Deep penetration; Magnetic resonance imaging; Nanospike; Self-assembly; Synergistic therapy; Tumor microenvironment-responsive.
© 2022 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Figures






Similar articles
-
Chemotherapeutic drug-photothermal agent co-self-assembling nanoparticles for near-infrared fluorescence and photoacoustic dual-modal imaging-guided chemo-photothermal synergistic therapy.J Control Release. 2017 Jul 28;258:95-107. doi: 10.1016/j.jconrel.2017.05.011. Epub 2017 May 10. J Control Release. 2017. PMID: 28501673
-
Polydopamine Nanoparticles as a Versatile Molecular Loading Platform to Enable Imaging-guided Cancer Combination Therapy.Theranostics. 2016 Apr 28;6(7):1031-42. doi: 10.7150/thno.14431. eCollection 2016. Theranostics. 2016. PMID: 27217836 Free PMC article.
-
Tumor microenvironment responsive theranostic agent for enhanced chemo/chemodynamic/photothermal therapy.Colloids Surf B Biointerfaces. 2022 Oct;218:112750. doi: 10.1016/j.colsurfb.2022.112750. Epub 2022 Aug 3. Colloids Surf B Biointerfaces. 2022. PMID: 35961116
-
Dual pH/reduction-responsive hybrid polymeric micelles for targeted chemo-photothermal combination therapy.Acta Biomater. 2018 Jul 15;75:371-385. doi: 10.1016/j.actbio.2018.05.026. Epub 2018 May 17. Acta Biomater. 2018. PMID: 29777957
-
A redox-activated theranostic nanoagent: toward multi-mode imaging guided chemo-photothermal therapy.Chem Sci. 2018 Jul 2;9(33):6749-6757. doi: 10.1039/c8sc02446d. eCollection 2018 Sep 7. Chem Sci. 2018. PMID: 30310607 Free PMC article.
Cited by
-
Regulating the microenvironment with nanomaterials: Potential strategies to ameliorate COVID-19.Acta Pharm Sin B. 2023 Feb 21;13(9):3638-58. doi: 10.1016/j.apsb.2023.02.010. Online ahead of print. Acta Pharm Sin B. 2023. PMID: 36846153 Free PMC article. Review.
-
Manganese-derived biomaterials for tumor diagnosis and therapy.J Nanobiotechnology. 2024 Jun 15;22(1):335. doi: 10.1186/s12951-024-02629-8. J Nanobiotechnology. 2024. PMID: 38879519 Free PMC article.
-
Magnetic Resonance Imaging in Breast Cancer Tissue In Vitro after PDT Therapy.Diagnostics (Basel). 2024 Mar 6;14(5):563. doi: 10.3390/diagnostics14050563. Diagnostics (Basel). 2024. PMID: 38473036 Free PMC article.
-
Shape Matters: Impact of Mesoporous Silica Nanoparticle Morphology on Anti-Tumor Efficacy.Pharmaceutics. 2024 May 8;16(5):632. doi: 10.3390/pharmaceutics16050632. Pharmaceutics. 2024. PMID: 38794294 Free PMC article.
-
Application of molecular dynamics simulation in self-assembled cancer nanomedicine.Biomater Res. 2023 May 4;27(1):39. doi: 10.1186/s40824-023-00386-7. Biomater Res. 2023. PMID: 37143168 Free PMC article. Review.
References
-
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Zhang H., Liu K., Li S.K., Xin X., Yuan S.L., Ma G.H., et al. Self-assembled minimalist multifunctional theranostic nanoplatform for magnetic resonance imaging-guided tumor photodynamic therapy. ACS Nano. 2018;12:8266–8276. - PubMed
-
- Qin Y., Tong F., Zhang W., Zhou Y., He S.Q., Xie R., et al. Self-delivered supramolecular nanomedicine with transformable shape for ferrocene-amplified photodynamic therapy of breast cancer and bone metastases. Adv Funct Mater. 2021;31:2104645.
LinkOut - more resources
Full Text Sources

