Selective transduction of cerebellar Purkinje and granule neurons using delivery of AAV-PHP.eB and AAVrh10 vectors at axonal terminal locations
- PMID: 36176957
- PMCID: PMC9513253
- DOI: 10.3389/fnmol.2022.947490
Selective transduction of cerebellar Purkinje and granule neurons using delivery of AAV-PHP.eB and AAVrh10 vectors at axonal terminal locations
Abstract
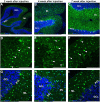
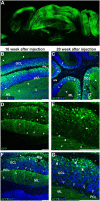
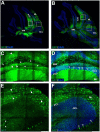
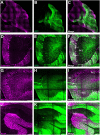
Adeno-associated virus (AAV)-based brain gene therapies require precision without off-targeting of unaffected neurons to avoid side effects. The cerebellum and its cell populations, including granule and Purkinje cells, are vulnerable to neurodegeneration; hence, conditions to deliver the therapy to specific cell populations selectively remain challenging. We have investigated a system consisting of the AAV serotypes, targeted injections, and transduction modes (direct or retrograde) for targeted delivery of AAV to cerebellar cell populations. We selected the AAV-PHP.eB and AAVrh10 serotypes valued for their retrograde features, and we thoroughly examined their cerebellar transduction pattern when injected into lobules and deep cerebellar nuclei. We found that AAVrh10 is suitable for the transduction of neurons in the mode highly dependent on placing the virus at axonal terminals. The strategy secures selective transduction for granule cells. The AAV-PHP.eB can transduce Purkinje cells and is very selective for the cell type when injected into the DCN at axonal PC terminals. Therefore, both serotypes can be used in a retrograde mode for selective transduction of major neuronal types in the cerebellum. Moreover, our in vivo transduction strategies are suitable for pre-clinical protocol development for gene delivery to granule cells by AAVrh10 and Purkinje cells by AAV-PHPeB.
Keywords: AAV-PHP.eB; AAVrh10; Purkinje cells; adeno-associated virus (AAV); cerebellum; deep cerebellar nuclei; granule cells; retrograde.
Copyright © 2022 Surdyka, Jesion, Niewiadomska-Cimicka, Trottier, Kalinowska-Pośka and Figiel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Selective transgene expression in cerebellar Purkinje cells and granule cells using adeno-associated viruses together with specific promoters.Brain Res. 2015 Sep 16;1620:1-16. doi: 10.1016/j.brainres.2015.05.015. Epub 2015 May 16. Brain Res. 2015. PMID: 25988836
-
Anatomical Evidence for a Direct Projection from Purkinje Cells in the Mouse Cerebellar Vermis to Medial Parabrachial Nucleus.Front Neural Circuits. 2018 Feb 7;12:6. doi: 10.3389/fncir.2018.00006. eCollection 2018. Front Neural Circuits. 2018. PMID: 29467628 Free PMC article.
-
Retrograde capabilities of adeno-associated virus vectors in the central nervous system.BioTechnologia (Pozn). 2021 Dec 22;102(4):473-478. doi: 10.5114/bta.2021.111111. eCollection 2021. BioTechnologia (Pozn). 2021. PMID: 36605599 Free PMC article. Review.
-
Pilot Study Assessing the Impact of Intrathecal Administration of Variants AAV-PHP.B and AAV-PHP.eB on Brain Transduction in Adult Rhesus Macaques.Front Bioeng Biotechnol. 2021 Nov 15;9:762209. doi: 10.3389/fbioe.2021.762209. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34869273 Free PMC article.
-
Modulatory Effects of Monoamines and Perineuronal Nets on Output of Cerebellar Purkinje Cells.Front Neural Circuits. 2021 Jun 14;15:661899. doi: 10.3389/fncir.2021.661899. eCollection 2021. Front Neural Circuits. 2021. PMID: 34194302 Free PMC article. Review.
Cited by
-
AAV-Mediated Restoration of Dystrophin-Dp71 in the Brain of Dp71-Null Mice: Molecular, Cellular and Behavioral Outcomes.Cells. 2024 Apr 20;13(8):718. doi: 10.3390/cells13080718. Cells. 2024. PMID: 38667332 Free PMC article.
-
AAV-delivered muscone-induced transgene system for treating chronic diseases in mice via inhalation.Nat Commun. 2024 Feb 6;15(1):1122. doi: 10.1038/s41467-024-45383-z. Nat Commun. 2024. PMID: 38321056 Free PMC article.
-
AAV-Mediated CAG-Targeting Selectively Reduces Polyglutamine-Expanded Protein and Attenuates Disease Phenotypes in a Spinocerebellar Ataxia Mouse Model.Int J Mol Sci. 2024 Apr 15;25(8):4354. doi: 10.3390/ijms25084354. Int J Mol Sci. 2024. PMID: 38673939 Free PMC article.
-
Genomic and epigenomic insights into purkinje and granule neurons in Alzheimer's disease and related dementia using single-nucleus multiome analysis.Res Sq [Preprint]. 2025 Apr 1:rs.3.rs-6264481. doi: 10.21203/rs.3.rs-6264481/v1. Res Sq. 2025. PMID: 40235507 Free PMC article. Preprint.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

