Pregestational diabetes alters cardiac structure and function of neonatal rats through developmental plasticity
- PMID: 36176990
- PMCID: PMC9514058
- DOI: 10.3389/fcvm.2022.919293
Pregestational diabetes alters cardiac structure and function of neonatal rats through developmental plasticity
Abstract
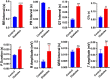
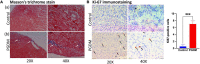
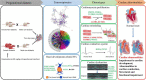
Pregestational diabetes (PGDM) leads to developmental impairment, especially cardiac dysfunction, in their offspring. The hyperglycemic microenvironment inside the uterus alters the cardiac plasticity characterized by electrical and structural remodeling of the heart. The altered expression of several transcription factors due to hyperglycemia during fetal development might be responsible for molecular defects and phenotypic changes in the heart. The molecular mechanism of the developmental defects in the heart due to PGDM remains unclear. To understand the molecular defects in the 2-days old neonatal rats, streptozotocin-induced diabetic female rats were bred with healthy male rats. We collected 2-day-old hearts from the neonates and identified the molecular basis for phenotypic changes. Neonates from diabetic mothers showed altered electrocardiography and echocardiography parameters. Transcriptomic profiling of the RNA-seq data revealed that several altered genes were associated with heart development, myocardial fibrosis, cardiac conduction, and cell proliferation. Histopathology data showed the presence of focal cardiac fibrosis and increased cell proliferation in neonates from diabetic mothers. Thus, our results provide a comprehensive map of the cellular events and molecular pathways perturbed in the neonatal heart during PGDM. All of the molecular and structural changes lead to developmental plasticity in neonatal rat hearts and develop cardiac anomalies in their early life.
Keywords: RNA sequencing; cardiac dysfunction; developmental plasticity; heart development; neonates; pregestational diabetes.
Copyright © 2022 Alam, Uppulapu, Tiwari, Varghese, Mohammed, Adela, Arava and Banerjee.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Shand AW, Bell JC, McElduff A, Morris J, Roberts CL. Outcomes of pregnancies in women with pre-gestational diabetes mellitus and gestational diabetes mellitus; a population-based study in New South Wales, Australia, 1998-2002. Diabet Med J Br Diabet Assoc. (2008) 25:708–15. 10.1111/j.1464-5491.2008.02431.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

