SARS-CoV-2 modulates virus receptor expression in placenta and can induce trophoblast fusion, inflammation and endothelial permeability
- PMID: 36177036
- PMCID: PMC9513489
- DOI: 10.3389/fimmu.2022.957224
SARS-CoV-2 modulates virus receptor expression in placenta and can induce trophoblast fusion, inflammation and endothelial permeability
Abstract
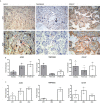
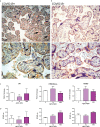
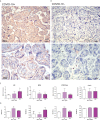
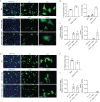
SARS-CoV-2 is a devastating virus that induces a range of immunopathological mechanisms including cytokine storm, apoptosis, inflammation and complement and coagulation pathway hyperactivation. However, how the infection impacts pregnant mothers is still being worked out due to evidence of vertical transmission of the SARS-CoV-2, and higher incidence of pre-eclampsia, preterm birth, caesarian section, and fetal mortality. In this study, we assessed the levels of the three main receptors of SARS-CoV-2 (ACE2, TMPRSS2 and CD147) in placentae derived from SARS-CoV-2 positive and negative mothers. Moreover, we measured the effects of Spike protein on placental cell lines, in addition to their susceptibility to infection. SARS-CoV-2 negative placentae showed elevated levels of CD147 and considerably low amount of TMPRSS2, making them non-permissive to infection. SARS-CoV-2 presence upregulated TMPRSS2 expression in syncytiotrophoblast and cytotrophoblast cells, thereby rendering them amenable to infection. The non-permissiveness of placental cells can be due to their less fusogenicity due to infection. We also found that Spike protein was capable of inducing pro-inflammatory cytokine production, syncytiotrophoblast apoptosis and increased vascular permeability. These events can elicit pre-eclampsia-like syndrome that marks a high percentage of pregnancies when mothers are infected with SARS-CoV-2. Our study raises important points relevant to SARS-CoV-2 mediated adverse pregnancy outcomes.
Keywords: ACE2; CD147; SARS-CoV-2; TMPRSS2; pregnancy.
Copyright © 2022 Agostinis, Toffoli, Spazzapan, Balduit, Zito, Mangogna, Zupin, Salviato, Maiocchi, Romano, Crovella, Fontana, Braga, Confalonieri, Ricci, Kishore and Bulla.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

