Antitumor effects of fecal microbiota transplantation: Implications for microbiome modulation in cancer treatment
- PMID: 36177041
- PMCID: PMC9513044
- DOI: 10.3389/fimmu.2022.949490
Antitumor effects of fecal microbiota transplantation: Implications for microbiome modulation in cancer treatment
Abstract
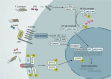
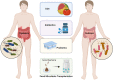
Fecal microbiome transplantation (FMT) from healthy donors is one of the techniques for restoration of the dysbiotic gut, which is increasingly being used to treat various diseases. Notably, mounting evidence in recent years revealed that FMT has made a breakthrough in the oncology treatment area, especially by improving immunotherapy efficacy to achieve antitumor effects. However, the mechanism of FMT in enhancing antitumor effects of immune checkpoint blockers (ICBs) has not yet been fully elucidated. This review systematically summarizes the role of microbes and their metabolites in the regulation of tumor immunity. We highlight the mechanism of action of FMT in the treatment of refractory tumors as well as in improving the efficacy of immunotherapy. Furthermore, we summarize ongoing clinical trials combining FMT with immunotherapy and further focus on refined protocols for the practice of FMT in cancer treatment, which could guide future directions and priorities of FMT scientific development.
Keywords: FMT; cancer; fecal microbiota transplantation; gut microbiota; immunotherapy.
Copyright © 2022 Xu, Cao, Ren, Weng, Liu, Guo, Wang, Han, Ren and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
From chaos to order: optimizing fecal microbiota transplantation for enhanced immune checkpoint inhibitors efficacy.Gut Microbes. 2025 Dec;17(1):2452277. doi: 10.1080/19490976.2025.2452277. Epub 2025 Jan 18. Gut Microbes. 2025. PMID: 39826104 Review.
-
Gut Microbiome Modulation Via Fecal Microbiota Transplant to Augment Immunotherapy in Patients with Melanoma or Other Cancers.Curr Oncol Rep. 2020 Jun 24;22(7):74. doi: 10.1007/s11912-020-00913-y. Curr Oncol Rep. 2020. PMID: 32577835 Free PMC article. Review.
-
Fecal microbiota transplantation in cancer management: Current status and perspectives.Int J Cancer. 2019 Oct 15;145(8):2021-2031. doi: 10.1002/ijc.32003. Epub 2018 Dec 30. Int J Cancer. 2019. PMID: 30458058 Free PMC article. Review.
-
Conversion of unresponsiveness to immune checkpoint inhibition by fecal microbiota transplantation in patients with metastatic melanoma: study protocol for a randomized phase Ib/IIa trial.BMC Cancer. 2022 Dec 30;22(1):1366. doi: 10.1186/s12885-022-10457-y. BMC Cancer. 2022. PMID: 36585700 Free PMC article.
-
Encyclopedia of fecal microbiota transplantation: a review of effectiveness in the treatment of 85 diseases.Chin Med J (Engl). 2022 Aug 20;135(16):1927-1939. doi: 10.1097/CM9.0000000000002339. Chin Med J (Engl). 2022. PMID: 36103991 Free PMC article.
Cited by
-
Cellular and Molecular Mechanisms of the Tumor Stroma in Colorectal Cancer: Insights into Disease Progression and Therapeutic Targets.Biomedicines. 2023 Aug 23;11(9):2361. doi: 10.3390/biomedicines11092361. Biomedicines. 2023. PMID: 37760801 Free PMC article. Review.
-
Updates in the pathogenesis and management of immune-related enterocolitis, hepatitis and cardiovascular toxicities.Immunooncol Technol. 2024 Jan 5;21:100704. doi: 10.1016/j.iotech.2024.100704. eCollection 2024 Mar. Immunooncol Technol. 2024. PMID: 38357008 Free PMC article. Review.
-
Fecal Microbiota Transplantation: Insights into Colon Carcinogenesis and Immune Regulation.J Clin Med. 2024 Nov 1;13(21):6578. doi: 10.3390/jcm13216578. J Clin Med. 2024. PMID: 39518717 Free PMC article. Review.
-
Effects of gut microbiota in breast cancer.Front Oncol. 2025 Jun 17;15:1617410. doi: 10.3389/fonc.2025.1617410. eCollection 2025. Front Oncol. 2025. PMID: 40599853 Free PMC article. Review.
-
A New Treatment Landscape for RCC: Association of the Human Microbiome with Improved Outcomes in RCC.Cancers (Basel). 2023 Feb 1;15(3):935. doi: 10.3390/cancers15030935. Cancers (Basel). 2023. PMID: 36765892 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

