Photochemical Mechanisms of Fluorophores Employed in Single-Molecule Localization Microscopy
- PMID: 36177530
- PMCID: PMC10100239
- DOI: 10.1002/anie.202204745
Photochemical Mechanisms of Fluorophores Employed in Single-Molecule Localization Microscopy
Abstract
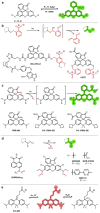
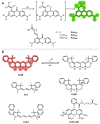
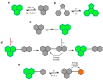
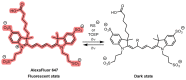
Decoding cellular processes requires visualization of the spatial distribution and dynamic interactions of biomolecules. It is therefore not surprising that innovations in imaging technologies have facilitated advances in biomedical research. The advent of super-resolution imaging technologies has empowered biomedical researchers with the ability to answer long-standing questions about cellular processes at an entirely new level. Fluorescent probes greatly enhance the specificity and resolution of super-resolution imaging experiments. Here, we introduce key super-resolution imaging technologies, with a brief discussion on single-molecule localization microscopy (SMLM). We evaluate the chemistry and photochemical mechanisms of fluorescent probes employed in SMLM. This Review provides guidance on the identification and adoption of fluorescent probes in single molecule localization microscopy to inspire the design of next-generation fluorescent probes amenable to single-molecule imaging.
Keywords: Fluorescence; Photochemistry; Photoswitching; Sensors; Super-Resolution.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
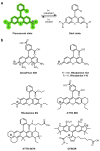
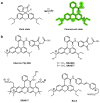
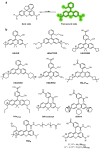
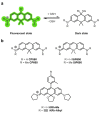

Similar articles
-
Chemistry of Photosensitive Fluorophores for Single-Molecule Localization Microscopy.ACS Chem Biol. 2019 Jun 21;14(6):1077-1090. doi: 10.1021/acschembio.9b00197. Epub 2019 May 13. ACS Chem Biol. 2019. PMID: 30997987 Review.
-
Improved resolution in single-molecule localization microscopy using QD-PAINT.Exp Mol Med. 2021 Mar;53(3):384-392. doi: 10.1038/s12276-021-00572-4. Epub 2021 Mar 2. Exp Mol Med. 2021. PMID: 33654221 Free PMC article.
-
Switchable Fluorophores for Single-Molecule Localization Microscopy.Chem Rev. 2018 Sep 26;118(18):9412-9454. doi: 10.1021/acs.chemrev.7b00767. Epub 2018 Sep 17. Chem Rev. 2018. PMID: 30221931 Free PMC article. Review.
-
Molecular resolution imaging by post-labeling expansion single-molecule localization microscopy (Ex-SMLM).Nat Commun. 2020 Jul 7;11(1):3388. doi: 10.1038/s41467-020-17086-8. Nat Commun. 2020. PMID: 32636396 Free PMC article.
-
[Comparison and progress review of various super-resolution fluorescence imaging techniques].Se Pu. 2021 Oct;39(10):1055-1064. doi: 10.3724/SP.J.1123.2021.06015. Se Pu. 2021. PMID: 34505427 Free PMC article. Review. Chinese.
Cited by
-
Photoactivatable BODIPYs for Live-Cell PALM.Molecules. 2023 Mar 7;28(6):2447. doi: 10.3390/molecules28062447. Molecules. 2023. PMID: 36985424 Free PMC article. Review.
-
Molecular mapping of neuronal architecture using STORM microscopy and new fluorescent probes for SMLM imaging.Neurophotonics. 2024 Jan;11(1):014414. doi: 10.1117/1.NPh.11.1.014414. Epub 2024 Mar 8. Neurophotonics. 2024. PMID: 38464866 Free PMC article.
-
Enhanced β-Amyloid Aggregation in Living Cells Imaged with Quinolinium-Based Spontaneous Blinking Fluorophores.Chem Biomed Imaging. 2023 Sep 27;2(1):56-63. doi: 10.1021/cbmi.3c00081. eCollection 2024 Jan 22. Chem Biomed Imaging. 2023. PMID: 39473459 Free PMC article.
-
A Photoactivatable Plasma Membrane Probe Based on a Self-Triggered Photooxidation Cascade for Live Cell Super-Resolution Microscopy.Angew Chem Int Ed Engl. 2025 Jun 10;64(24):e202425276. doi: 10.1002/anie.202425276. Epub 2025 Apr 21. Angew Chem Int Ed Engl. 2025. PMID: 40192285 Free PMC article.
-
Fundamental Limits in Measuring the Anisotropic Rotational Diffusion of Single Molecules.J Phys Chem A. 2024 Jul 18;128(28):5808-5815. doi: 10.1021/acs.jpca.4c03160. Epub 2024 Jul 8. J Phys Chem A. 2024. PMID: 38978460 Free PMC article.
References
-
- None
-
- Williams R. M., Zipfel W. R., Webb W. W., Curr. Opin. Chem. Biol. 2001, 5, 603–608; - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

