Process Dependent Complexity in Multicomponent Gels
- PMID: 36177680
- PMCID: PMC11475255
- DOI: 10.1002/marc.202200709
Process Dependent Complexity in Multicomponent Gels
Abstract
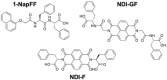
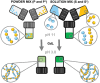
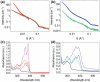
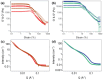
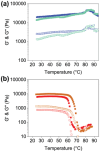
Mixing low molecular weight gelators (LMWGs) can be used to combine favorable properties of the individual components within a multifunctional gel. Such multicomponent systems are complex enough in themselves but the method of combining components is not commonly considered something to influence self-assembly. Herein, two multicomponent systems comprising of a naphthalene-based dipeptide hydrogelator and one of two modified naphthalene diimides (NDIs), one of which forms gels, and the other does not, are investigated. These systems are probed, examining the structures formed and their gel properties (when preparing a solution from either a mixed powder of both components or by mixing pre-formed solutions of each component) using rheology, small angle neutron scattering (SANS), and absorbance spectroscopy. It is found that by altering the method of preparation, it is can either induce self-sorting or co-assembly within the fibers formed that underpin the gel network.
Keywords: co-assembly; gels; low molecular weight gelators; self-assembly; self-sorting.
© 2022 The Authors. Macromolecular Rapid Communications published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Draper E. R., Adams D. J., Chem 2017, 3, 390.
-
- Terech P., Weiss R. G., Chem. Rev. 1997, 97, 3133. - PubMed
-
- Weiss R. G., J. Am. Chem. Soc. 2014, 136, 7519. - PubMed
-
- Draper E. R., Greeves B. J., Barrow M., Schweins R., Zwijnenburg M. A., Adams D. J., Chem 2017, 2, 716.
-
- Draper E. R., Mears L. L. E. E., Castilla A. M., King S. M., Mcdonald T. O., Akhtar R., Adams D. J., RSC Adv. 2015, 5, 95369.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

