Activation of Ge-H and Sn-H Bonds with N-Heterocyclic Carbenes and a Cyclic (Alkyl)(amino)carbene
- PMID: 36177710
- PMCID: PMC10100474
- DOI: 10.1002/chem.202202493
Activation of Ge-H and Sn-H Bonds with N-Heterocyclic Carbenes and a Cyclic (Alkyl)(amino)carbene
Abstract
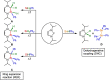
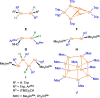
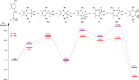
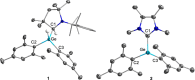
A study of the reactivity of several N-heterocyclic carbenes (NHCs) and the cyclic (alkyl)(amino)carbene 1-(2,6-di-iso-propylphenyl)-3,3,5,5-tetramethyl-pyrrolidin-2-ylidene (cAACMe ) with the group 14 hydrides GeH2 Mes2 and SnH2 Me2 (Me=CH3 , Mes=1,3,5-(CH3 )3 C6 H2 ) is presented. The reaction of GeH2 Mes2 with cAACMe led to the insertion of cAACMe into one Ge-H bond to give cAACMe H-GeHMes2 (1). If 1,3,4,5-tetramethyl-imidazolin-2-ylidene (Me2 ImMe ) was used as the carbene, NHC-mediated dehydrogenative coupling occurred, which led to the NHC-stabilized germylene Me2 ImMe ⋅GeMes2 (2). The reaction of SnH2 Me2 with cAACMe also afforded the insertion product cAACMe H-SnHMe2 (3), and reaction of two equivalents Me2 ImMe with SnH2 Me2 gave the NHC-stabilized stannylene Me2 ImMe ⋅SnMe2 (4). If the sterically more demanding NHCs Me2 ImMe , 1,3-di-isopropyl-4,5-dimethyl-imidazolin-2-ylidene (iPr2 ImMe ) and 1,3-bis-(2,6-di-isopropylphenyl)-imidazolin-2-ylidene (Dipp2 Im) were employed, selective formation of cyclic oligomers (SnMe2 )n (5; n=5-8) in high yield was observed. These cyclic oligomers were also obtained from the controlled decomposition of cAACMe H-SnHMe2 (3).
Keywords: N-heterocyclic carbenes; cyclic alkyl(amino)carbenes; germanium; hydrides; tin.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
N-heterocyclic carbene and cyclic (alkyl)(amino)carbene adducts of plumbanes and plumbylenes.Dalton Trans. 2022 Sep 13;51(35):13488-13498. doi: 10.1039/d2dt02462d. Dalton Trans. 2022. PMID: 35997066
-
Reactivity of NHC Alane Adducts towards N-Heterocyclic Carbenes and Cyclic (Alkyl)(amino)carbenes: Ring Expansion, Ring Opening, and Al-H Bond Activation.Chemistry. 2017 Sep 7;23(50):12387-12398. doi: 10.1002/chem.201702166. Epub 2017 Jul 21. Chemistry. 2017. PMID: 28603878
-
How to Decarbonize N-Heterocyclic Carbenes (NHCs): The simple Alane Adducts (NHC) ⋅ AlR3 (R=H, Me, Et).Angew Chem Int Ed Engl. 2024 May 27;63(22):e202403639. doi: 10.1002/anie.202403639. Epub 2024 Mar 25. Angew Chem Int Ed Engl. 2024. PMID: 38446008
-
Development of six-membered saturated cyclic diaminocarbenes in main-group chemistry.Dalton Trans. 2025 Jun 10;54(23):9113-9124. doi: 10.1039/d5dt00648a. Dalton Trans. 2025. PMID: 40259828 Review.
-
NHC aluminum chemistry on the rise.Dalton Trans. 2024 Oct 15;53(40):16436-16454. doi: 10.1039/d4dt01660b. Dalton Trans. 2024. PMID: 39225565 Review.
Cited by
-
Carbene-activated stannylenes to access selective C(sp3)-H bond scission at the steric limit.Nat Commun. 2025 Mar 18;16(1):2657. doi: 10.1038/s41467-025-57907-2. Nat Commun. 2025. PMID: 40102441 Free PMC article.
-
An easy-to-perform evaluation of steric properties of Lewis acids.Chem Sci. 2023 Feb 6;14(9):2275-2288. doi: 10.1039/d3sc00037k. eCollection 2023 Mar 1. Chem Sci. 2023. PMID: 36873848 Free PMC article.
References
-
- None
-
- Herrmann W. A., Köcher C., Angew. Chem. Int. Ed. 1997, 36, 2162–2187;
- Angew. Chem. 1997, 109, 2256–2282;
-
- Power P. P., Chem. Rev. 1999, 99, 3463–3504; - PubMed
-
- Bourissou D., Guerret O., Gabbai F. P., Bertrand G., Chem. Rev. 2000, 100, 39–92; - PubMed
-
- Hahn F. E., Jahnke M. C., Angew. Chem. Int. Ed. 2008, 47, 3122–3172; - PubMed
- Angew. Chem. 2008, 120, 3166–3216;
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

