Assessment of flomoxef combined with amikacin in a hollow-fibre infection model for the treatment of neonatal sepsis in low- and middle-income healthcare settings
- PMID: 36177766
- PMCID: PMC9704437
- DOI: 10.1093/jac/dkac323
Assessment of flomoxef combined with amikacin in a hollow-fibre infection model for the treatment of neonatal sepsis in low- and middle-income healthcare settings
Abstract
Background: Annual mortality from neonatal sepsis is an estimated 430 000-680 000 infants globally, most of which occur in low- and middle-income countries (LMICs). The WHO currently recommends a narrow-spectrum β-lactam (e.g. ampicillin) and gentamicin as first-line empirical therapy. However, available epidemiological data demonstrate high rates of resistance to both agents. Alternative empirical regimens are needed. Flomoxef and amikacin are two off-patent antibiotics with potential for use in this setting.
Objectives: To assess the pharmacodynamics of flomoxef and amikacin in combination.
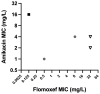
Methods: The pharmacodynamic interaction of flomoxef and amikacin was assessed in chequerboard assays and a 16-arm dose-ranged hollow-fibre infection model (HFIM) experiment. The combination was further assessed in HFIM experiments mimicking neonatal plasma exposures of clinically relevant doses of both drugs against five Enterobacterales isolates with a range of flomoxef/amikacin MICs.
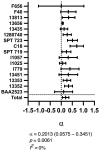
Results: Flomoxef and amikacin in combination were synergistic in bacterial killing in both assays and prevention of emergence of amikacin resistance in the HFIM. In the HFIM assessing neonatal-like drug exposures, the combination killed 3/5 strains to sterility, (including 2/5 that monotherapy with either drug failed to kill) and failed to kill the 2/5 strains with flomoxef MICs of 32 mg/L.
Conclusions: We conclude that the combination of flomoxef and amikacin is synergistic and is a potentially clinically effective regimen for the empirical treatment of neonatal sepsis in LMIC settings and is therefore suitable for further assessment in a clinical trial.
© The Author(s) 2022. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures

Similar articles
-
Flomoxef and fosfomycin in combination for the treatment of neonatal sepsis in the setting of highly prevalent antimicrobial resistance.J Antimicrob Chemother. 2022 Apr 27;77(5):1334-1343. doi: 10.1093/jac/dkac038. J Antimicrob Chemother. 2022. PMID: 35170719 Free PMC article.
-
Amikacin Combined with Fosfomycin for Treatment of Neonatal Sepsis in the Setting of Highly Prevalent Antimicrobial Resistance.Antimicrob Agents Chemother. 2021 Jun 17;65(7):e0029321. doi: 10.1128/AAC.00293-21. Epub 2021 Jun 17. Antimicrob Agents Chemother. 2021. PMID: 33972238 Free PMC article.
-
Flomoxef for neonates: extending options for treatment of neonatal sepsis caused by ESBL-producing Enterobacterales.J Antimicrob Chemother. 2022 Feb 23;77(3):711-718. doi: 10.1093/jac/dkab468. J Antimicrob Chemother. 2022. PMID: 34969066 Free PMC article.
-
Potential Antibiotics for the Treatment of Neonatal Sepsis Caused by Multidrug-Resistant Bacteria.Paediatr Drugs. 2021 Sep;23(5):465-484. doi: 10.1007/s40272-021-00465-z. Epub 2021 Aug 26. Paediatr Drugs. 2021. PMID: 34435316 Free PMC article. Review.
-
Antibiotic regimens for late-onset neonatal sepsis.Cochrane Database Syst Rev. 2021 May 8;5(5):CD013836. doi: 10.1002/14651858.CD013836.pub2. Cochrane Database Syst Rev. 2021. PMID: 33998665 Free PMC article.
Cited by
-
An Overview of Antibiotic Therapy for Early- and Late-Onset Neonatal Sepsis: Current Strategies and Future Prospects.Antibiotics (Basel). 2024 Mar 10;13(3):250. doi: 10.3390/antibiotics13030250. Antibiotics (Basel). 2024. PMID: 38534685 Free PMC article. Review.
-
Small-Molecule Antibiotic Drug Development: Need and Challenges.ACS Infect Dis. 2023 Nov 10;9(11):2062-2071. doi: 10.1021/acsinfecdis.3c00189. Epub 2023 Oct 11. ACS Infect Dis. 2023. PMID: 37819866 Free PMC article. Review.
-
Real-World Use, Effectiveness, and Safety of Intravenous Fosfomycin: The FORTRESS Study.Infect Dis Ther. 2025 Apr;14(4):765-791. doi: 10.1007/s40121-025-01125-2. Epub 2025 Mar 19. Infect Dis Ther. 2025. PMID: 40106180 Free PMC article.

