Microbial succession in a marine sediment: Inferring interspecific microbial interactions with marine cable bacteria
- PMID: 36178156
- PMCID: PMC10092204
- DOI: 10.1111/1462-2920.16230
Microbial succession in a marine sediment: Inferring interspecific microbial interactions with marine cable bacteria
Abstract
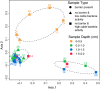
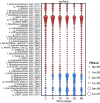
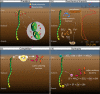
Cable bacteria are long, filamentous, multicellular bacteria that grow in marine sediments and couple sulfide oxidation to oxygen reduction over centimetre-scale distances via long-distance electron transport. Cable bacteria can strongly modify biogeochemical cycling and may affect microbial community networks. Here we examine interspecific interactions with marine cable bacteria (Ca. Electrothrix) by monitoring the succession of 16S rRNA amplicons (DNA and RNA) and cell abundance across depth and time, contrasting sediments with and without cable bacteria growth. In the oxic zone, cable bacteria activity was positively associated with abundant predatory bacteria (Bdellovibrionota, Myxococcota, Bradymonadales), indicating putative predation on cathodic cells. At suboxic depths, cable bacteria activity was positively associated with sulfate-reducing and magnetotactic bacteria, consistent with cable bacteria functioning as ecosystem engineers that modify their local biogeochemical environment, benefitting certain microbes. Cable bacteria activity was negatively associated with chemoautotrophic sulfur-oxidizing Gammaproteobacteria (Thiogranum, Sedimenticola) at oxic depths, suggesting competition, and positively correlated with these taxa at suboxic depths, suggesting syntrophy and/or facilitation. These observations are consistent with chemoautotrophic sulfur oxidizers benefitting from an oxidizing potential imparted by cable bacteria at suboxic depths, possibly by using cable bacteria as acceptors for electrons or electron equivalents, but by an as yet enigmatic mechanism.
© 2022 The Authors. Environmental Microbiology published by Society for Applied Microbiology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bazylinski, D.A. , Williams, T.J. , Lefèvre, C.T. , Trubitsyn, D. , Fang, J. , Beveridge, T.J. et al. (2013) Magnetovibrio blakemorei gen. nov., sp. nov., a magnetotactic bacterium (Alphaproteobacteria: Rhodospirillaceae) isolated from a salt marsh. International Journal of Systematic and Evolutionary Microbiology, 63, 1824–1833. 10.1099/ijs.0.044453-0 - DOI - PubMed
-
- Berg, P. , Risgaard‐Petersen, N. & Rysgaard, S. (1998) Interpretation of measured concentration profiles in the sediment porewater. Limnology and Oceanography, 43, 1500–1510. 10.4319/lo.1998.43.7.1500 - DOI
-
- Bjerg, J.T. , Boschker, H.T.S. , Larsen, S. , Berry, D. , Schmid, M. , Millo, D. et al. (2018) Long‐distance electron transport in individual, living cable bacteria. Proceedings of the National Academy of Sciences of the United States of America, 115, 5786–5791. 10.1073/pnas.1800367115 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

