Engineering Cancer Selective Virotherapies: Are the Pieces of the Puzzle Falling into Place?
- PMID: 36178346
- PMCID: PMC9700347
- DOI: 10.1089/hum.2022.178
Engineering Cancer Selective Virotherapies: Are the Pieces of the Puzzle Falling into Place?
Abstract
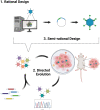
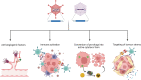
Advances in gene therapy, synthetic biology, cancer genomics, and patient-derived cancer models have expanded the repertoire of strategies for targeting human cancers using viral vectors. Novel capsids, synthetic promoters, and therapeutic payloads are being developed and assessed through approaches such as rational design, pooled library screening, and directed evolution. Ultimately, the goal is to generate precision-engineered viruses that target different facets of tumor cell biology, without compromising normal tissue and organ function. In this study, we briefly review the opportunities for engineering cancer selectivity into viral vectors at both the cell extrinsic and intrinsic level. Such stringently tumor-targeted vectors can subsequently act as platforms for the delivery of potent therapeutic transgenes, including the exciting prospect of immunotherapeutic payloads. These have the potential to eradicate nontransduced cells through stimulation of systemic anticancer immune responses, thereby side-stepping the inherent challenge of achieving gene delivery to the entire cancer cell population. We discuss the importance of using advanced primary human cellular models, such as patient-derived cultures and organoids, to enable rapid screening and triage of novel candidates using disease-relevant models. We believe this combination of improved delivery and selectivity, through novel capsids and promoters, coupled with more potent choices for the combinations of immunotherapy-based payloads seems capable of finally delivering innovative new gene therapies for oncology. Many pieces of the puzzle of how to build a virus capable of targeting human cancers appear to be falling into place.
Keywords: AAV; adenovirus; cancer; capsid engineering; directed evolution; enhancer; gene therapy; immunotherapy; oncolytics; promoter; rational design; therapeutic payloads.
Conflict of interest statement
A.L.P. is Chief Scientific Officer of Trocept Therapeutics, a wholly owned subsidiary of Accession Therapeutics Ltd. All other authors have no competing financial interests.
Figures

Similar articles
-
Small But Increasingly Mighty: Latest Advances in AAV Vector Research, Design, and Evolution.Hum Gene Ther. 2017 Nov;28(11):1075-1086. doi: 10.1089/hum.2017.172. Epub 2017 Aug 23. Hum Gene Ther. 2017. PMID: 28835125 Review.
-
Adeno-associated virus (AAV) vectors in cancer gene therapy.J Control Release. 2016 Oct 28;240:287-301. doi: 10.1016/j.jconrel.2016.01.001. Epub 2016 Jan 12. J Control Release. 2016. PMID: 26796040 Free PMC article. Review.
-
Adeno-Associated Virus Engineering and Load Strategy for Tropism Modification, Immune Evasion and Enhanced Transgene Expression.Int J Nanomedicine. 2024 Jul 29;19:7691-7708. doi: 10.2147/IJN.S459905. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39099791 Free PMC article. Review.
-
Vector Affinity and Receptor Distribution Define Tissue-Specific Targeting in an Engineered AAV Capsid.J Virol. 2023 Jun 29;97(6):e0017423. doi: 10.1128/jvi.00174-23. Epub 2023 May 18. J Virol. 2023. PMID: 37199615 Free PMC article.
-
Engineering and evolution of synthetic adeno-associated virus (AAV) gene therapy vectors via DNA family shuffling.J Vis Exp. 2012 Apr 2;(62):3819. doi: 10.3791/3819. J Vis Exp. 2012. PMID: 22491297 Free PMC article.
Cited by
-
Oncolytic Virus Engineering and Utilizations: Cancer Immunotherapy Perspective.Viruses. 2023 Jul 28;15(8):1645. doi: 10.3390/v15081645. Viruses. 2023. PMID: 37631987 Free PMC article. Review.
-
Channeling the Natural Properties of Sindbis Alphavirus for Targeted Tumor Therapy.Int J Mol Sci. 2023 Oct 6;24(19):14948. doi: 10.3390/ijms241914948. Int J Mol Sci. 2023. PMID: 37834397 Free PMC article. Review.
-
Oncolytic virotherapy in cancer treatment: challenges and optimization prospects.Front Immunol. 2023 Dec 15;14:1308890. doi: 10.3389/fimmu.2023.1308890. eCollection 2023. Front Immunol. 2023. PMID: 38169820 Free PMC article. Review.
-
An αvβ6-specific virotherapy expressing bispecific immune cell activators induces immune cell activation to mediate tumor cell death.Mol Ther Oncol. 2025 Jun 25;33(3):201017. doi: 10.1016/j.omton.2025.201017. eCollection 2025 Sep 18. Mol Ther Oncol. 2025. PMID: 40741595 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
