Population Pharmacokinetics and Exposure-Response Analyses for Risankizumab in Patients with Active Psoriatic Arthritis
- PMID: 36178584
- PMCID: PMC9561470
- DOI: 10.1007/s40744-022-00495-0
Population Pharmacokinetics and Exposure-Response Analyses for Risankizumab in Patients with Active Psoriatic Arthritis
Abstract
Introduction: Risankizumab is an anti-IL23 monoclonal antibody approved for the treatment of moderate to severe plaque psoriasis and active psoriatic arthritis (PsA). This work characterizes the pharmacokinetics of risankizumab in PsA compared with psoriasis and evaluates the efficacy and safety exposure-response relationships in PsA.
Methods: The population pharmacokinetic analyses included data from 1527 participants that originated from one phase 1 healthy participant study, one phase 2 dose-ranging study in patients with PsA with an open-label extension study, and two pivotal phase 3 studies in patients with PsA, where the clinical regimen of risankizumab 150 mg administered subcutaneously (SC) at weeks 0, 4, and every 12 weeks thereafter was compared with placebo. Pharmacokinetics were analyzed using nonlinear mixed-effects modeling. Simulation analyses using the final model were conducted to evaluate the impact of covariates on exposure. Data from 1407 patients with PsA from the phase 3 studies were included in the exposure-response analyses. Graphical analyses were used to evaluate efficacy and safety exposure-response relationships, and logistic regression was conducted for further assessment of efficacy exposure-response relationships.
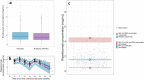
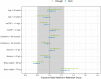
Results: Risankizumab pharmacokinetics were well described by a two-compartment model with first-order SC absorption and elimination. None of the evaluated covariates showed clinically relevant impact on exposure. On the basis of the final model, systemic clearance, steady-state volume of distribution, and terminal phase elimination half-life were estimated to be ~ 0.31 L/day, 11.1 L, and 26.3 days, respectively, for a typical 90 kg patient with PsA. Absolute SC bioavailability was estimated to be 83.5%. Exposure-response quartile analyses suggested that exposures associated with the clinical regimen maximized efficacy across the endpoints evaluated. No exposure dependency was observed for key safety endpoints.
Conclusions: Risankizumab exhibited linear and time-independent pharmacokinetics in patients with PsA and was comparable to patients with plaque psoriasis. Efficacy and safety exposure-response analyses support that the clinical regimen achieved robust efficacy with a favorable safety profile for patients with active PsA.
Clinical trials: NCT02596217, NCT02719171, NCT02986373, NCT03671148, and NCT03675308.
Clinical trials: NCT02596217, NCT02719171, NCT02986373, NCT03671148, and NCT03675308.
Keywords: ACR20/50/70; Efficacy; Minimal disease activity; PASI 90/100; Pharmacokinetics; Psoriatic arthritis; Risankizumab.
© 2022. The Author(s).
Figures


References
-
- SKYRIZI® (risankizumab-rzaa) injection, for subcutaneous use. [United States package insert]. North Chicago, IL; AbbVie Inc. 2019.
-
- Kristensen LE, Keiserman M, Papp K, McCasland L, White D, Lu W, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 1 trial. Ann Rheum Dis. 2022;81(2):225–231. doi: 10.1136/annrheumdis-2021-221019. - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

