Epidemiology Evidence for Health Effects of 150 per- and Polyfluoroalkyl Substances: A Systematic Evidence Map
- PMID: 36178797
- PMCID: PMC9524599
- DOI: 10.1289/EHP11185
Epidemiology Evidence for Health Effects of 150 per- and Polyfluoroalkyl Substances: A Systematic Evidence Map
Abstract
Background: Per- and polyfluoroalkyl substances (PFAS) comprise a large class of chemicals with widespread use and persistence in the environment and in humans; however, most of the epidemiology research has focused on a small subset.
Objectives: The aim of this systematic evidence map (SEM) is to summarize the epidemiology evidence on approximately 150 lesser studied PFAS prioritized by the EPA for tiered toxicity testing, facilitating interpretation of those results as well as identification of priorities for risk assessment and data gaps for future research.
Methods: The Populations, Exposure, Comparators, and Outcomes (PECO) criteria were intentionally broad to identify studies of any health effects in humans with information on associations with exposure to the identified PFAS. Systematic review methods were used to search for literature that was screened using machine-learning software and manual review. Studies meeting the PECO criteria underwent quantitative data extraction and evaluation for risk of bias and sensitivity using the Integrated Risk Information System approach.
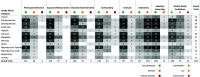
Results: 193 epidemiology studies were identified, which included information on 15 of the PFAS of interest. The most commonly studied health effect categories were metabolic (), endocrine (), cardiovascular (30), female reproductive (), developmental (), immune (), nervous (), male reproductive (), cancer (), and urinary () effects. In study evaluation, 120 (62%) studies were considered High/Medium confidence for at least one outcome.
Discussion: Most of the PFAS in this SEM have little to no epidemiology data available to inform evaluation of potential health effects. Although exposure to the 15 PFAS that had data was fairly low in most studies, these less-studied PFAS may be used as replacements for "legacy" PFAS, leading to potentially greater exposure. It is impractical to generate epidemiology evidence to fill the existing gaps for all potentially relevant PFAS. This SEM highlights some of the important research gaps that currently exist. https://doi.org/10.1289/EHP11185.
Figures
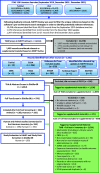
![Figure 1A is a tabular representation titled Study evaluation domains having seven rows and two columns, namely, Epidemiology domain and Core question. Row 1: Exposure measurement. Does the exposure measure reliably distinguish between levels of exposure in a time window considered most relevant for a causal effect with respect to the development of the outcome? Row 2: Outcome ascertainment. Does the outcome measure reliably distinguish the presence or absence (or degree of severity) of the outcome? Row 3: Participant selection. Is there evidence that selection into or out of the study (or analysis sample) was jointly related to exposure and to outcome? [note: this includes attrition]. Row 4: Confounding. Is confounding of the effect of the exposure likely? Row 5: Analysis. Does the analysis strategy and presentation convey the necessary familiarity with the data and assumptions? Row 6: Sensitivity. Is there a concern that sensitivity of the study is not adequate to detect an effect? Row 7: Selective reporting. Is there reason to be concerned about selective reporting? Figure 1B is a tabular representation titled Domain judgments having three rows and two columns, namely, judgment and interpretation. Row 1: Good implies appropriate study conduct relating to the domain and minor deficiencies not expected to influence results. Adequate implies a study that may have some limitations relating to the domain, but they are not likely to be severe or to have a notable impact on results. Row 2: Deficient implies identified biases or deficiencies interpreted as likely to have a notable impact on the results or prevent reliable interpretation of study findings. Row 3: Critically deficient implies a serious flaw identified that makes the observes effect(s) uninterpretable. Studies with a critical deficiency will almost always be considered “uninformative” overall. Figure 1C is a tabular representation titled Overall study rating for an outcome having three rows and two columns, namely, Rating and Interpretation. Row 1: High implies no notable deficiencies or concerns identified; potential for bias unlikely or minimal; sensitive methodology. Medium implies possible deficiencies or concerns noted but resulting bias or lack of sensitivity is unlikely to be a notable degree. Row 2: Low implies deficiencies or concerns were noted, and the potential for substantive bias or inadequate sensitivity could have a significant impact on the study results or their interpretation. Row 3: Uninformative implies serious flaw(s) makes study results unusable for hazard identification or dose response.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/ea5c/9524599/2aacdfece8bd/ehp11185_f1.gif)


References
-
- U.S. Centers for Disease Control and Prevention. 2019. Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, January 2019, vol. 2. Atlanta, GA: U.S. Department of Health and Human Services.
-
- U.S. EPA (U.S. Environmental Protection Agency). 2016. Health effects support document for perfluorooctanoic acid (PFOA). Washington, DC: U.S. Environmental Protection Agency, Office of Water, Health and Ecological Criteria Division.
-
- U.S. EPA. 2021. Risk management for per- and polyfluoroalkyl substances (PFAS) under TSCA. https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/risk-man... [accessed 1 March 2022].
-
- U.S. EPA. CompTox Chemicals Dashboard: PFAS Master List of PFAS Substances (Version 4). https://comptox.epa.gov/dashboard/chemical_lists/PFASMASTER [accessed 8 August 2021].
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

