Widespread employment of conserved C. elegans homeobox genes in neuronal identity specification
- PMID: 36178933
- PMCID: PMC9524666
- DOI: 10.1371/journal.pgen.1010372
Widespread employment of conserved C. elegans homeobox genes in neuronal identity specification
Abstract
Homeobox genes are prominent regulators of neuronal identity, but the extent to which their function has been probed in animal nervous systems remains limited. In the nematode Caenorhabditis elegans, each individual neuron class is defined by the expression of unique combinations of homeobox genes, prompting the question of whether each neuron class indeed requires a homeobox gene for its proper identity specification. We present here progress in addressing this question by extending previous mutant analysis of homeobox gene family members and describing multiple examples of homeobox gene function in different parts of the C. elegans nervous system. To probe homeobox function, we make use of a number of reporter gene tools, including a novel multicolor reporter transgene, NeuroPAL, which permits simultaneous monitoring of the execution of multiple differentiation programs throughout the entire nervous system. Using these tools, we add to the previous characterization of homeobox gene function by identifying neuronal differentiation defects for 14 homeobox genes in 24 distinct neuron classes that are mostly unrelated by location, function and lineage history. 12 of these 24 neuron classes had no homeobox gene function ascribed to them before, while in the other 12 neuron classes, we extend the combinatorial code of transcription factors required for specifying terminal differentiation programs. Furthermore, we demonstrate that in a particular lineage, homeotic identity transformations occur upon loss of a homeobox gene and we show that these transformations are the result of changes in homeobox codes. Combining the present with past analyses, 113 of the 118 neuron classes of C. elegans are now known to require a homeobox gene for proper execution of terminal differentiation programs. Such broad deployment indicates that homeobox function in neuronal identity specification may be an ancestral feature of animal nervous systems.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

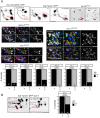





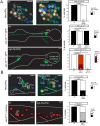
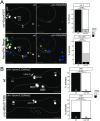



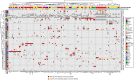
References
-
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philosophical Transactions of the Royal Society of London B Biological Sciences. 1986;314:1–340. - PubMed
-
- Glenwinkel L, Taylor SR, Langebeck-Jensen K, Pereira L, Reilly MB, Basavaraju M, et al.. In silico analysis of the transcriptional regulatory logic of neuronal identity specification throughout the C. elegans nervous system. eLife. 2021;10. Epub 2021/06/25. doi: 10.7554/eLife.64906 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

