TLR4 activation induces inflammatory vascular permeability via Dock1 targeting and NOX4 upregulation
- PMID: 36179995
- PMCID: PMC12203677
- DOI: 10.1016/j.bbadis.2022.166562
TLR4 activation induces inflammatory vascular permeability via Dock1 targeting and NOX4 upregulation
Abstract
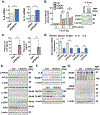
The loss of vascular integrity is a cardinal feature of acute inflammatory responses evoked by activation of the TLR4 inflammatory cascade. Utilizing in vitro and in vivo models of inflammatory lung injury, we explored TLR4-mediated dysregulated signaling that results in the loss of endothelial cell (EC) barrier integrity and vascular permeability, focusing on Dock1 and Elmo1 complexes that are intimately involved in regulation of Rac1 GTPase activity, a well recognized modulator of vascular integrity. Marked reductions in Dock1 and Elmo1 expression was observed in lung tissues (porcine, rat, mouse) exposed to TLR4 ligand-mediated acute inflammatory lung injury (LPS, eNAMPT) in combination with injurious mechanical ventilation. Lung tissue levels of Dock1 and Elmo1 were preserved in animals receiving an eNAMPT-neutralizing mAb in conjunction with highly significant decreases in alveolar edema and lung injury severity, consistent with Dock1/Elmo1 as pathologic TLR4 targets directly involved in inflammation-mediated loss of vascular barrier integrity. In vitro studies determined that pharmacologic inhibition of Dock1-mediated activation of Rac1 (TBOPP) significantly exacerbated TLR4 agonist-induced EC barrier dysfunction (LPS, eNAMPT) and attenuated increases in EC barrier integrity elicited by barrier-enhancing ligands of the S1P1 receptor (sphingosine-1-phosphate, Tysiponate). The EC barrier-disrupting influence of Dock1 inhibition on S1PR1 barrier regulation occurred in concert with: 1) suppressed formation of EC barrier-enhancing lamellipodia, 2) altered nmMLCK-mediated MLC2 phosphorylation, and 3) upregulation of NOX4 expression and increased ROS. These studies indicate that Dock1 is essential for maintaining EC junctional integrity and is a critical target in TLR4-mediated inflammatory lung injury.
Keywords: Dock1; EC barrier integrity; Elmo1; Oxidative stress; Vascular permeability; eNAMPT.
Copyright © 2022. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest Joe GN Garcia, MD is CEO and founder of Aqualung Therapeutics Corporation. All other authors declare no competing financial interests.
Figures
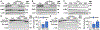
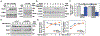

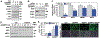
Similar articles
-
Inflammatory lung injury is associated with endothelial cell mitochondrial fission and requires the nitration of RhoA and cytoskeletal remodeling.Free Radic Biol Med. 2024 Aug 20;221:125-135. doi: 10.1016/j.freeradbiomed.2024.05.019. Epub 2024 May 10. Free Radic Biol Med. 2024. PMID: 38734269 Free PMC article.
-
4-Octyl itaconate alleviates endothelial cell inflammation and barrier dysfunction in LPS-induced sepsis via modulating TLR4/MAPK/NF-κB signaling : 4-Octyl itaconate alleviates endothelial dysfunction.Mol Med. 2025 Jun 16;31(1):240. doi: 10.1186/s10020-025-01160-2. Mol Med. 2025. PMID: 40524158 Free PMC article.
-
Music intervention mitigates LPS-induced gut barrier disruption and immune stress in broilers via TLR4/NF-κB regulation.Poult Sci. 2025 Jul;104(7):105189. doi: 10.1016/j.psj.2025.105189. Epub 2025 Apr 22. Poult Sci. 2025. PMID: 40294553 Free PMC article.
-
Electronic cigarettes for smoking cessation.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD010216. doi: 10.1002/14651858.CD010216.pub7. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 Jan 8;1:CD010216. doi: 10.1002/14651858.CD010216.pub8. PMID: 36384212 Free PMC article. Updated.
-
Electronic cigarettes for smoking cessation.Cochrane Database Syst Rev. 2021 Sep 14;9(9):CD010216. doi: 10.1002/14651858.CD010216.pub6. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 Nov 17;11:CD010216. doi: 10.1002/14651858.CD010216.pub7. PMID: 34519354 Free PMC article. Updated.
Cited by
-
A Razor's Edge: Vascular Responses to Acute Inflammatory Lung Injury/Acute Respiratory Distress Syndrome.Annu Rev Physiol. 2024 Feb 12;86:505-529. doi: 10.1146/annurev-physiol-042222-030731. Annu Rev Physiol. 2024. PMID: 38345908 Free PMC article. Review.
-
Research Progress on the Immune Function of Liver Sinusoidal Endothelial Cells in Sepsis.Cells. 2025 Mar 4;14(5):373. doi: 10.3390/cells14050373. Cells. 2025. PMID: 40072101 Free PMC article. Review.
-
Vaginal lactobacilli produce anti-inflammatory β-carboline compounds.Cell Host Microbe. 2024 Nov 13;32(11):1897-1909.e7. doi: 10.1016/j.chom.2024.09.014. Epub 2024 Oct 17. Cell Host Microbe. 2024. PMID: 39423813
-
eNAMPT/TLR4 inflammatory cascade activation is a key contributor to SLE Lung vasculitis and alveolar hemorrhage.J Transl Autoimmun. 2022 Dec 22;6:100181. doi: 10.1016/j.jtauto.2022.100181. eCollection 2023. J Transl Autoimmun. 2022. PMID: 36619655 Free PMC article.
-
DOCK1 deficiency drives placental trophoblast cell dysfunction by influencing inflammation and oxidative stress, hallmarks of preeclampsia.Hypertens Res. 2024 Dec;47(12):3434-3446. doi: 10.1038/s41440-024-01920-3. Epub 2024 Oct 8. Hypertens Res. 2024. PMID: 39379467
References
-
- Dudek SM, Garcia JG, Cytoskeletal regulation of pulmonary vascular permeability, J. Appl. Physiol 91 (2001) (1985) 1487–1500. - PubMed
-
- Thompson BT, Chambers RC, Liu KD, Acute respiratory distress syndrome, N. Engl. J. Med 377 (2017) 562–572. - PubMed
-
- Hong SB, Huang Y, Moreno-Vinasco L, Sammani S, Moitra J, Barnard JW, Ma SF, Mirzapoiazova T, Evenoski C, Reeves RR, Chiang ET, Lang GD, Husain AN, Dudek SM, Jacobson JR, Ye SQ, Lussier YA, Garcia JG, Essential role of pre-B-cell colony enhancing factor in ventilator-induced lung injury, Am. J. Respir. Crit. Care Med 178 (2008) 605–617. - PMC - PubMed
-
- Bajwa EK, Yu CL, Gong MN, Thompson BT, Christiani DC, Pre-B-cell colony-enhancing factor gene polymorphisms and risk of acute respiratory distress syndrome, Crit. Care Med 35 (2007) 1290–1295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

