A randomized placebo-controlled trial of convalescent plasma for adults hospitalized with COVID-19 pneumonia
- PMID: 36180450
- PMCID: PMC9523654
- DOI: 10.1038/s41598-022-19629-z
A randomized placebo-controlled trial of convalescent plasma for adults hospitalized with COVID-19 pneumonia
Abstract
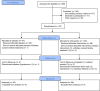
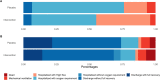
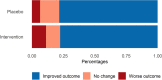
Passive immunotherapy with convalescent plasma may be the only available agent during the early phases of a pandemic. Here, we report safety and efficacy of high-titer convalescent plasma for COVID-19 pneumonia. Double-blinded randomized multicenter placebo-controlled trial of adult patients hospitalized with COVID-19 pneumonia. The intervention was COVID-19 convalescent plasma and placebo was saline allocated 2:1. The primary outcome was clinical status 14 days after the intervention evaluated on a clinical ordinal scale. The trial was registered at ClinicalTrials.Gov, NCT04345289, 14/04/2020. The CCAP-2 trial was terminated prematurely due to futility. Of 147 patients randomized, we included 144 patients in the modified intention-to-treat population. The ordinal clinical status 14 days post-intervention was comparable between treatment groups (odds ratio (OR) 1.41, 95% confidence interval (CI) 0.72-2.09). Results were consistent when evaluating clinical progression on an individual level 14 days after intervention (OR 1.09; 95% CI 0.46-1.73). No significant differences in length of hospital stay, admission to ICU, frequency of severe adverse events or all-cause mortality during follow-up were found between the intervention and the placebo group. Infusion of convalescent plasma did not influence clinical progression, survival or length of hospitalization in patients with COVID-19 pneumonia.
© 2022. The Author(s).
Conflict of interest statement
Dr Nielsen declares grants from Novo Nordisk Foundation and personal fees from GSK and MDS outside of the submitted work. Dr Østergaard reports personal fees from Sanofi. Dr Erikstrup reports grants from Abbots diagnostic outside of the submitted work. Dr Benfield reports grants from Novo Nordisk Foundation, grants from Simonsen Foundation, grants and personal fees from GSK, grants and personal fees from Pfizer, personal fees from Boehringer Ingelheim, personal fees from Abbvie, grants and personal fees from Gilead, personal fees from MSD, grants from Lundbeck Foundation, grants from Kai Hansen Foundation, and grants from Erik and Susanna Olsen’s Charitable Fund outside the submitted work. All other authors have nothing to disclose.
Figures
References
-
- Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw F-M, Lim WS, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta-analysis. J. Infect. Dis. 2015;211(1):80–90. doi: 10.1093/infdis/jiu396. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical