How mechanisms of stem cell polarity shape the human cerebral cortex
- PMID: 36180551
- PMCID: PMC10571506
- DOI: 10.1038/s41583-022-00631-3
How mechanisms of stem cell polarity shape the human cerebral cortex
Abstract
Apical-basal progenitor cell polarity establishes key features of the radial and laminar architecture of the developing human cortex. The unique diversity of cortical stem cell populations and an expansion of progenitor population size in the human cortex have been mirrored by an increase in the complexity of cellular processes that regulate stem cell morphology and behaviour, including their polarity. The study of human cells in primary tissue samples and human stem cell-derived model systems (such as cortical organoids) has provided insight into these processes, revealing that protein complexes regulate progenitor polarity by controlling cell membrane adherence within appropriate cortical niches and are themselves regulated by cytoskeletal proteins, signalling molecules and receptors, and cellular organelles. Studies exploring how cortical stem cell polarity is established and maintained are key for understanding the features of human brain development and have implications for neurological dysfunction.
© 2022. Springer Nature Limited.
Conflict of interest statement
Competing interests
A.R.K. is a co-founder, consultant and member of the Board of Neurona Therapeutics. The other authors declare no competing interests.
Figures

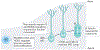
References
-
- Götz M & Huttner WB The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol 6, 777–788 (2005). - PubMed

