The cytoprotective role of GM1 ganglioside in Huntington disease cells
- PMID: 36180805
- PMCID: PMC9712292
- DOI: 10.1007/s11033-022-07830-2
The cytoprotective role of GM1 ganglioside in Huntington disease cells
Abstract
Background: Huntington disease (HD) is a neurodegenerative disease where a genetic mutation leads to excessive polyglutamine (Q) repeats in the huntingtin protein. The polyglutamine repeats create toxic plaques when the protein is cleaved, leading to neuron death. The glycolipid GM1 ganglioside (GM1) has been shown to be neuroprotective in HD models, as it prevents the cleavage of the mutant huntingtin protein by phosphorylation of serine 13 and 16. Previous studies have tested GM1 in both adult-onset and juvenile-onset HD models, but this study set out to investigate whether GM1 mediated cytoprotection is influenced by the length of polyglutamine repeats.
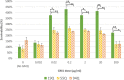
Method and result: This study utilized cell culture to analyze the effect of GM1 on cell viability, directly comparing the response between cells with adult-onset HD and juvenile-onset HD. HEK293 cells expressing either wild-type huntingtin (Htt) (19Q) exon 1, adult-onset HD mutant Htt exon 1 (55Q), or Juvenile HD mutant Htt exon 1 (94Q) were assessed for cell viability using the WST-1 assay. Our results suggested moderate doses of GM1 increased cell viability for all cell lines when compared to untreated cells. When comparing HEK293 55Q and 94Q cells, there was no difference in cell viability within each dose of GM1.
Conclusion: These data suggest cellular responses to GM1 are independent of polyglutamine repeats in HD cells and provide insight on GM1's application as a therapeutic agent for HD and other diseases.
Keywords: Cytoprotection; GM1 ganglioside; Huntington’s disease; Juvenile Huntington’s disease; Mutant huntingtin; Polyglutamine repeats.
© 2022. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

