A c-Fos activation map in nitroglycerin/levcromakalim-induced models of migraine
- PMID: 36180824
- PMCID: PMC9524028
- DOI: 10.1186/s10194-022-01496-8
A c-Fos activation map in nitroglycerin/levcromakalim-induced models of migraine
Abstract
Background: Chronic migraine is a common and highly disabling disorder. Functional MRI has indicated that abnormal brain region activation is linked with chronic migraine. Drugs targeting the calcitonin gene-related peptide (CGRP) or its receptor have been reported to be efficient for treating chronic migraine. The CGRP signaling was also shared in two types of chronic migraine models (CMMs). However, it remains unclear whether the activation of specific brain regions could contribute to persistent behavioral sensitization, and CGRP receptor antagonists relieve migraine-like pain in CMMs by altering specific brain region activation. Therefore, it's of great interest to investigate brain activation pattern and the effect of olcegepant (a CGRP receptor-specific antagonist) treatment on alleviating hyperalgesia by altering brain activation in two CMMs, and provide a reference for future research on neural circuits.
Methods: Repeated administration of nitroglycerin (NTG) or levcromakalim (LEV) was conducted to stimulate human migraine-like pain and establish two types of CMMs in mice. Mechanical hypersensitivity was evaluated by using the von Frey filament test. Then, we evaluated the activation of different brain regions with c-Fos and NeuN staining. Olcegepant was administered to explore its effect on mechanical hyperalgesia and brain region activation.
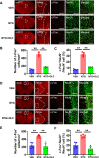
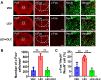
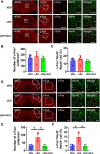
Results: In two CMMs, acute and basal mechanical hyperalgesia was observed, and olcegepant alleviated mechanical hyperalgesia. In the NTG-induced CMM, the medial prefrontal cortex (mPFC), anterior cingulate cortex (ACC), and the caudal part of the spinal trigeminal nucleus (Sp5c) showed a significant increase of c-Fos expression in the NTG group (p < 0.05), while pre-treatment with olcegepant reduced c-Fos expression compared with NTG group (p < 0.05). No significant difference of c-Fos expression was found in the paraventricular thalamic nucleus (PVT) and ventrolateral periaqueductal gray (vlPAG) between the vehicle control and NTG group (p > 0.05). In the LEV-induced CMM, mPFC, PVT, and Sp5c showed a significant increase of c-Fos expression between vehicle control and LEV group, and olcegepant reduced c-Fos expression (p < 0.05). No significant difference in c-Fos expression was found in vlPAG and ACC (p > 0.05).
Conclusions: Our study demonstrated the activation of mPFC and Sp5c in two CMMs. Olcegepant may alleviate hyperalgesia of the hind paw and periorbital area by attenuating brain activation in CMMs.
Keywords: CGRP; Chronic migraine; Migraine-like pain; c-Fos.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Maniyar FH, Sprenger T, Monteith T, Schankin C, Goadsby PJ (2014) Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain 137(Pt 1): 232–241. 10.1093/brain/awt320 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

